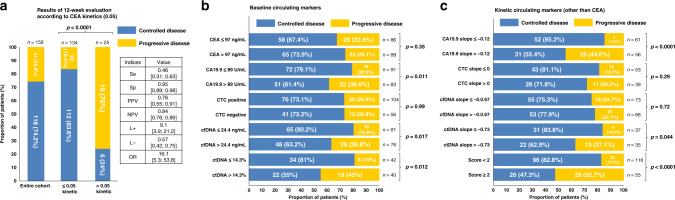
Fig. 2. Result of biomarkers for prediction of progression at 12-weeks evaluation.
Prediction of progression according to (a) 0.05 threshold CEA kinetics value previously identified (primary endpoint), (b) baseline circulating marker median value and (c) kinetics circulating marker median value. CA19-9, carbohydrate antigen 19-9; CEA, Carcinoembryonic antigen; cfDNA, cell-free DNA; CTC, circulating tumour cells; ctDNA, circulating tumour DNA; L-, likelihood negative ratio; L+, likelihood positive ratio; NPV, negative predictive value; OR, odds ratio; PPV, positive predictive value; Se, sensitivity; Sp, specificity.