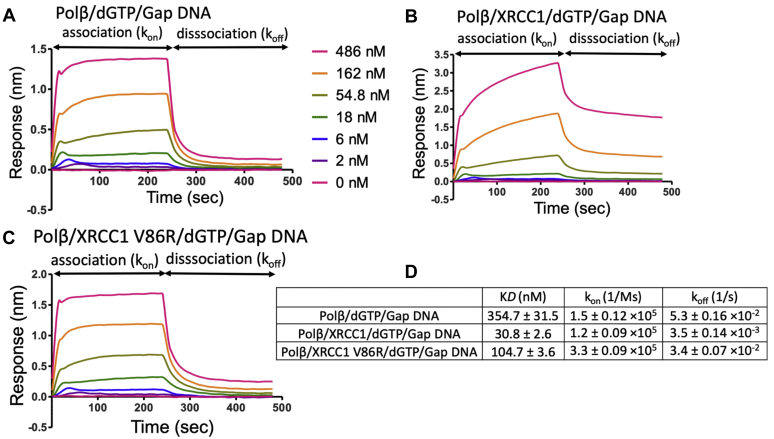
Figure 5.
Gap DNA-binding kinetics of polβ/XRCC1 complex in the presence of dGTP. The real-time DNA-binding kinetics of polβ/dGTP to one nucleotide gap DNA with template base C in the absence (A) and presence (B) wild-type XRCC1 and polβ/XRCC1 interaction mutant V86R (C). D, table shows the effect of XRCC1 on the equilibrium binding constant (KD), the association (kon) and dissociation (koff) rates of polβ/dGTP/gap DNA catalytic ternary complex. The data are processed and analyzed with ForteBio data analysis software with 1:1 binding model.