Abstract
Data of the osmotic water permeability of a lipid bilayer (diphytanoylphosphaticylcholin) in the presence of cholesterol (30 mole%) are shown under the simultaneous measurement of bilayer tension. Detailed methods and procedures for evaluating the water permeability using the moving membrane method (K. Yano, M. Iwamoto, T. Koshiji & S. Oiki: Visualizing the Osmotic Water Permeability of a Lipid Bilayer under Measured Bilayer Tension Using a Moving Membrane Method. Journal of Membrane Science, 627 (2021) 119231) are presented. The planar lipid bilayer is formed in a glass capillary, separating two aqueous compartments with different osmolarities, and osmotically-driven water flux is visualized as membrane movements along the capillary. The water permeability was evaluated under constant membrane area and tension after correcting for the unstirred layer effect. In these measurements, geometrical features, such as the edge of the planar lipid bilayer and the contact angle between bilayer and monolayer, were image-analyzed. The unstirred layer was evaluated electrophysiologically, in which gramicidin A channel was employed. In the presence of an osmotic gradient, the gramicidin channel generates the streaming potential, and the measured streaming potential data and the derived water-ion coupling ratio (water flux/ion flux) are shown. Detailed descriptions of the integrated method of the moving membrane allow researchers to reproduce the experiment and give opportunities to examine water permeability of various types of membranes, including those containing aquaporins. The present data of osmotic water permeability are compared with the previously published data, while they neglected the bilayer tension.
Keywords: Water permeability, Bilayer tension, Unstirred layer, Lipid bilayer, Ion channel, Streaming potential, Gramicidin channel
Specifications Table
| Subject | Physiology, Biophysics; Physical Sciences, Surfaces and Interfaces |
| Specific subject area | Osmotic water permeability of lipid bilayer under a measured bilayer tension |
| Type of data | Table Image Fig. |
| How data were acquired | Electrophysiological methods Image analysis Physicochemical calculations |
| Data format | Raw Analyzed |
| Parameters for data collection | The moving velocity of a lipid bilayer formed in a glass capillary under an osmotic gradient is measured by inspecting bilayer images. The osmotic water permeability for a measured bilayer area and tension is evaluated after correcting the unstirred layer. The streaming potential generated in the presence of the gramicidin channel was evaluated electrophysiologically. |
| Description of data collection | A lipid bilayer of diphytanoylphosphatidylcholine and cholesterol (30 mole%) formed in a glass capillary is subjected to move upon application of an osmotic gradient, and the velocity of the membrane was measured under a microscope (moving membrane method). From the membrane geometry, the bilayer area and tension were evaluated using an integrated method of image analysis and electrophysiology. Also, the unstirred layer was evaluated by a method using the gramicidin channel as a probe, and the streaming potential generated by the gramicidin channel was measured. Accordingly, osmotic water permeability of lipid bilayer in a fixed bilayer area and tension was evaluated. |
| Data source location | Institution: University of Fukui City/Town/Region: Fukui Country: Japan |
| Data accessibility | With the article |
| Related research article | Keita Yano, Masayuki Iwamoto, Takaaki Koshiji, Shigetoshi Oiki, Visualizing the Osmotic Water Permeability of a Lipid Bilayer under Measured Bilayer Tension Using a Moving Membrane Method. Journal of Membrane Science 627 (2021) 119231, https://doi.org/10.1016/j.memsci.2021.119231 |
Value of the Data
-
•
The osmotic water permeability (Pf) of the lipid bilayer is an essential parameter for evaluating water flux across the cell membrane, fundamental for various cellular activities. However, evaluation is complex owing to numerous factors affecting the measurements. The moving membrane (MM) method circumvents previous problems and establishes it as an accurate and simple method for evaluating the Pf.
-
•
Researchers in biological and physicochemical studies on the water permeability through membranes would benefit by acquiring an accurate and easy evaluation method and data thereby. The MM method is promising for evaluating the Pf of lipid bilayers with variable lipid compositions and can be extended for evaluating the Pf of aquaporins embedded therein.
-
•
The Pf data presented here is an unprecedented reference under a measured membrane tension. The streaming potential data are necessary for evaluating the unstirred layer, but it is also valid for inferring molecular mechanisms of ion permeation through channel molecules.
1. Data Description
1.1. Raw data and their calculation for the Pf values
In the moving membrane method, the velocity of the moving membrane, velm, is measured visually (Table 1, lower), from which Pf is evaluated as [1]
| (1) |
where Acap is the capillary cross-sectional area (µm2), vw is the partial molar volume of water (cm3/mol), velm is the membrane velocity (µm/s), Amem is the membrane area (µm2), and Δcs is the osmotic gradient (mOsm/L) (Table 1). In the Pf evaluation, correction of unstirred layer (UL) is a prerequisite, which is evaluated as follows. Both compartments contain the same NaCl concentration, and the current ratio of the gramicidin channel at ±100 mV, I+100/I-100, was measured (Table 1). I+100/I-100 represents a concentration difference of permeating Na+ ions at the channel entrance on both sides. Given a linear relationship of concentration and current amplitude at the measured concentration range, the polarization ratio (Table 1) indicates the local concentration polarization of permeating Na+ across the membrane. This polarization contributes to the osmotic gradient (ΔOsmelectrolyte = [electrolyte bulk concentration] × [polarization ratio]). Urea is added to only one side of the compartments; thus, the concentration polarization contributed by urea is half of the polarization ratio obtained by currents through the gramicidin channel (ΔOsmurea = [urea concentration] × [polarization ratio]/2)[1]. Accordingly, the osmotic gradient is the sum of the local concentration of urea and polarized concentration of Na+.
Table 1.
Raw data to calculate the Pf values of DPhPC membrane. (Upper) Data for the UL correction. (Lower) Raw data of the membrane movements. Pf is calculated from Eq. (1) such that Jv/Amem is divided by vw and ΔOsm multiplied by 106. These data are plotted in the reference [1]Fig. 3B.
| Osmolarity | Polarization | UL corrected | Capillary | Capillary | Partial molar | |||
|---|---|---|---|---|---|---|---|---|
| of urea | I+100 / | ratio (I+100 / | Osmotic gradient | radius | cross sectional | volume of water | ||
| (mOsm/L) | I-100 | I-100 - 1) | (mOsm/L) | (mm) | area (mm2) | (vw: cm3/mol) | ||
| 200 | 1.018 | 0.0185 | 194.465 | 0.55 | 0.9503 | 18 | ||
| 400 | 1.04 | 0.04005 | 391.99 | |||||
| 600 | 1,061 | 0.06105 | 581.685 | |||||
| 800 | 1.078 | 0.077913 | 768.835 | |||||
| 1000 | 1.105 | 0.10541 | 947.295 | |||||
| †100 mM NaCl both sides. | ||||||||
| Osmolarity | Experi- | Membrane | Elapsed | Membrane | Volume | Membrane | Membrane | |
| of urea | mental | movement | time | velocity | flux | diameter | area | Jv/ |
| (mOsm/ L) | number | distance (µm) | (sec) | (velm: µm/s) | (Jv: µm3/s) | (µm) | (A: µm2) | A (µm/s) |
| 200 | 1 | 21.9 | 690 | 0.03181 | 30,229 | 498 | 194,683 | 0.1553 |
| 2 | 33.63 | 690 | 0.04874 | 46,318 | 620 | 301,754 | 0.1535 | |
| 3 | 26.90 | 690 | 0.03899 | 37,052 | 575 | 259,541 | 0.1428 | |
| 4 | 21.95 | 690 | 0.03181 | 30,229 | 566 | 251,479 | 0.1202 | |
| 5 | 17.7 | 690 | 0.02565 | 24,375 | 490 | 188,479 | 0.1293 | |
| 6 | 26.55 | 690 | 0.03848 | 36,568 | 578 | 262,256 | 0.1394 | |
| 7 | 23.01 | 690 | 0.03335 | 31,693 | 524 | 215,542 | 0.147 | |
| 8 | 30. 8 | 690 | 0.04463 | 42,412 | 524 | 215,542 | 0.1968 | |
| mean | 25.305 | 690 | 0.036683 | 34,859.5 | 546.9 | 23,6159.5 | 0.14804 | |
| S.D. | 1.967 | 0 | 0.002848 | 2706.37 | 16.999 | 14,714.2 | 0.00868 | |
| 400 | 1 | 30.09 | 690 | 0.04361 | 41,443 | 497 | 193,902 | 0.2137 |
| 2 | 31.51 | 690 | 0.04566 | 43,391 | 472 | 174,885 | 0.2481 | |
| 3 | 42.13 | 690 | 0.06105 | 58,016 | 491 | 189,249 | 0.3066 | |
| 4 | 48.5 | 690 | 0.07029 | 66,797 | 539 | 228,059 | 0.2929 | |
| 5 | 31.15 | 690 | 0.04515 | 42,906 | 429 | 144,472 | 0.297 | |
| 6 | 43.54 | 690 | 0.0631 | 59,964 | 524 | 215,542 | 0.2782 | |
| 7 | 41.06 | 690 | 0.05951 | 56,552 | 502 | 197,823 | 0.2859 | |
| mean | 38.283 | 690 | 0.055481 | 52,724.1 | 493.49 | 191,990.3 | 0.2746 | |
| S.D. | 2.974 | 0 | 0.00431 | 4095.7 | 14.64 | 11,119.807 | 0.013346 | |
| 600 | 1 | 14.51 | 163 | 0.08904 | 84,615 | 593 | 276,044 | 0.3065 |
| 2 | 25.13 | 254 | 0.09895 | 94,032 | 519 | 211,448 | 0.4447 | |
| 3 | 29.03 | 526 | 0.05519 | 52,447 | 395 | 122,480 | 0.4282 | |
| 4 | 9.91 | 175 | 0.05664 | 53,825 | 475 | 177,116 | 0.3039 | |
| 5 | 20.18 | 292 | 0.0691 | 65,666 | 454 | 161801 | 0.4058 | |
| 6 | 49.21 | 655 | 0.07512 | 71,387 | 483 | 183,132 | 0.3898 | |
| mean | 24.662 | 344.2 | 0.074007 | 70,328.7 | 486.5 | 188,670.2 | 0.37982 | |
| S.D. | 6.204 | 89.9 | 0.007821 | 7432.2 | 29.6 | 23,169.2 | 0.02718 | |
| 800 | 1 | 60.18 | 690 | 0.08722 | 82,885 | 485 | 184,652 | 0.4489 |
| 2 | 35.4 | 690 | 0.0513 | 48,750 | 348 | 95,067 | 0.5128 | |
| 3 | 111.16 | 690 | 0.1611 | 153,093 | 614 | 295,942 | 0.5173 | |
| 4 | 49.21 | 690 | 0.07131 | 67,766 | 458 | 164,665 | 0.4115 | |
| 5 | 76.49 | 690 | 0.1109 | 105,388 | 569 | 254,152 | 0.4147 | |
| 6 | 82.13 | 690 | 0.119 | 113,086 | 525 | 216,366 | 0.5227 | |
| 7 | 52.04 | 690 | 0.07542 | 71,672 | 443 | 154,055 | 0.4652 | |
| 8 | 54.52 | 690 | 0.079 | 75,074 | 435 | 148,542 | 0.5054 | |
| mean | 65.14 | 690 | 0.094406 | 89714.3 | 484.6 | 189,180.1 | 0.47481 | |
| S.D. | 9.015 | 0 | 0.013067 | 12,417.1 | 31.6 | 24,211.0 | 0.01742 | |
| Osmolarity | Experi- | Membrane | Elapsed | Membrane | Volume | Membrane | Membrane | |
| of urea | mental | movement | time | velocity | flux | diameter | area | Jv/ |
| (mOsm/ L) | number | distance (µm) | (sec) | (velm: µm/s) | (Jv: µm3/s) | (µm) | (A: µm2) | A (µm/s) |
| 1000 | 1 | 63.72 | 573 | 0.1112 | 105,673 | 502 | 197,823 | 0.5342 |
| 2 | 59.12 | 690 | 0.08568 | 81,422 | 459 | 165,385 | 0.4923 | |
| 3 | 65.49 | 690 | 0.09491 | 90,193 | 446 | 156,149 | 0.5776 | |
| 4 | 87.08 | 690 | 0.1262 | 119,928 | 545 | 233,165 | 0.5143 | |
| 5 | 68.32 | 690 | 0.09902 | 94,099 | 438 | 150,598 | 0.6248 | |
| 6 | 71.15 | 690 | 0.1031 | 97,976 | 455 | 162,515 | 0.6029 | |
| 7 | 75.76 | 690 | 0.1098 | 104,343 | 504 | 199,403 | 0.5233 | |
| 8 | 105.85 | 690 | 0.1534 | 145,776 | 552 | 239,193 | 0.6094 | |
| mean | 74.561 | 675.4 | 0.110414 | 104,926 | 487.6 | 188,028.9 | 0.55985 | |
| S.D. | 5.760 | 15.6 | 0.007997 | 7599.3 | 16.9 | 13,140.9 | 0.01888 | |
1.2. Streaming potential of the gramicidin A channel at different osmolarity
The Vstream was measured at different ΔOsm, and the slope value was obtained as -5.66 ± 0.60 mV/ΔOsm (n=17) for K+ (Fig. 1). Ion permeates through the gramicidin channel via the single-file mode, and Vstream was related to the water-ion coupling ratio (CRw-i; water flux/ion flux) [2], [3], [4] as follows:
| (1) |
where vw is the partial water volume (Table 1), z is the valence of the current carrier, and F is the Faraday constant. The CRw-i value, thus obtained, was 12.3, which is consistent with earlier reports on the gramicidin A channel [4,5].
Fig. 1.
The streaming potential as a function of osmotic gradients.
The Vstream is used for correcting the current-voltage curve of the gramicidin channel [1].
1.3. Pf value of DPhPC with cholesterol
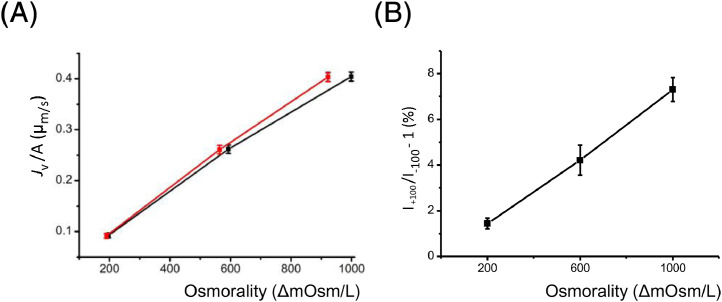
The Pf value of a diphytanoylphosphocholine (DPhPC) membrane with 30 mole% cholesterol was evaluated. The I+100/I-100 value was measured at the osmotic gradients (Fig. 2B; Table 2). Measured Jv /A values were plotted as a function of the UL-uncorrected (black) and -corrected (red) osmotic gradient. The Pf value of 26.2 ± 0.31 µm/s was obtained through fitting the linear function to the UL-corrected data (Fig. 2A). In parallel, bilayer tension was evaluated as 1.44 ± 0.16 mN/m using a previously described method [6].
Fig. 2.
Pf of a phosphatidylcholine membrane with 30 mole% cholesterol. A. Jv/A as a function of the osmotic gradient without (black) and with the UL correction (red). The Pf value was calculated as 26.20 ± 0.92 µm/s. B. The polarization ratio (I+100/I-100 - 1) at different osmotic gradient.
Table 2.
Raw data to calculate the Pf values of DPhPC with 30 mol% cholesterol. (Upper) Data for the UL correction. (Lower) Raw data of the membrane movements. Pf is calculated from Eq. (1) such that Jv/Amem is divided by vw and ΔOsm multiplied by 106. These data are plotted in Fig. 2.
| Osmolarity | Polarization | UL corrected | Capillary | Capillary | Partial molar | |||
|---|---|---|---|---|---|---|---|---|
| of urea | I+100 / | ratio (I+100 / | Osmotic gradient | radius | cross sectional | volume of water | ||
| (mOsm/L) | I-100 | I-100 - 1) | (mOsm/L) | (mm) | area (mm2) | (vw: cm3/mol) | ||
| 200 | 1.014 | 0.014 | 195.671 | 0.55 | 0.9503 | 18 | ||
| 600 | 1 | 0.042 | 587.364 | |||||
| 1000 | 1.073 | 0.073 | 963.48 | |||||
| †100 mM NaCl both sides. | ||||||||
| Osmolarity | Experi- | Membrane | Elapsed | Membrane | Volume | Membrane | Membrane | |
| of urea | mental | movement | time | velocity | flux | diameter | area | Jv/ |
| (mOsm/ L) | number | distance (µm) | (sec) | (velm: µm/s) | (Jv: µm3/s) | (µm) | (A: µm2) | A (µm/s) |
| 200 | 1 | 5.65 | 374 | 0.01511 | 14,355 | 426.4 | 142,714 | 0.1006 |
| 2 | 2.47 | 236 | 0.01047 | 9953 | 352.3 | 97,457 | 0.1021 | |
| 3 | 2.82 | 456 | 0.00619 | 5887 | 305.7 | 73,376 | 0.0802 | |
| 4 | 7.42 | 690 | 0.01075 | 10,212 | 375.7 | 110,776 | 0.0922 | |
| mean | 4.590 | 439 | 0.01063 | 10,101 | 365.03 | 106,080 | 0.09378 | |
| S.D. | 1.362 | 109.9 | 0.002101 | 1996.9 | 28.97 | 16,693 | 0.0058 | |
| 600 | 1 | 13.08 | 351 | 0.03727 | 35,416 | 394.848 | 122,385 | 0.2894 |
| 2 | 34.16 | 690 | 0.0495 | 47,042 | 456.543 | 163,619 | 0.2875 | |
| 3 | 1.82 | 70 | 0.02595 | 24,665 | 346.863 | 94,446 | 0.2612 | |
| 4 | 7.27 | 199 | 0.03652 | 34,704 | 415.413 | 135,466 | 0.2562 | |
| mean | 14.080 | 327.5 | 0.037311 | 35,456.8 | 403.41675 | 128,979 | 0.27358 | |
| S.D. | 8.171 | 154.4 | 0.00556 | 5283.6 | 26.327127 | 16,593.237 | 0.00999 | |
| 1000 | 1 | 41.30 | 642 | 0.06433 | 61,135 | 436.435 | 149,523 | 0.4089 |
| 2 | 53.69 | 690 | 0.07781 | 73,947 | 457 | 163,946 | 0.451 | |
| 3 | 40.55 | 690 | 0.05877 | 55,848 | 399.418 | 125,235 | 0.4459 | |
| 4 | 53.32 | 690 | 0.07727 | 73,430 | 461.113 | 166,911 | 0.4399 | |
| mean | 47.215 | 678 | 0.069546 | 66,090 | 438.4915 | 151,403.75 | 0.43643 | |
| S.D. | 4.197 | 13.9 | 0.005491 | 5218.12 | 16.279723 | 10,985.463 | 0.01091 | |
The previously published data, while they neglected the bilayer tension, are shown (Table 3).
Table 3.
Pf data from references. Historically accumulated Pf data, while the membrane tension is not evaluated.
| Pf value (µm/s) | Lipid composition | Membrane | Method | Authors | Journal |
|---|---|---|---|---|---|
| 19 | egg PC | PLB | osmotic flow method | Hanai and Haydon | J. Theoret. Biol. (1966) 11, 370-382 |
| 44 | PC | liposome | stopped flow | Reeves and Dowben | J. Membrane Biol. (1970) 3, 123-141 |
| 35-40 | Egg PC, DOPC | liposome | osmotic method | Fettiplace | BBA (1978) 513, 1-10 |
| 37 | GMO | PLB | Osmotic flow method | Dani and Levitt | Biophys. J. (1981) 35, 485-500 |
| 0.08∼0.3 | DMPC,DPPC and other | liposome | stopped flow | Jansen and Blume | Biophys. J. (1995) 68, 997-1008 |
| 14.9±1.7 | DphPC | liposome and PLB | chamber and light scattering | Hilmar, Zeidel | JBC (1996) 271 11627-11630 |
| 122∼662 | PC (18:0, 18:1, 18:3, 22:6) | liposome | light scattering | Huster et al. | Biophys. J. (1997) 73, 855-864 |
| 35±5 | egg PC | liposome | stopped flow | Dordas | J. Membrane Biol. (2000) 175, 95-105 |
| 34.4±3.5 | PE+PS+PI+Chol | PLB | ion selective electrode | Krylov et al. | J. Gen. Physiol. (2001) 118, 333-339 |
| 21∼158 | DLPE, DOPS, and other | liposome | stopped flow | Mathai et al. | J. Gen. Physiol. (2008) 131, 69-76 |
| 12 | DphPC | PLB | DIB | Dixit et al. | Langmuir (2012) 28, 7442-7451 |
| 42±3 | DphPC | PLB | DIB | Milianta et al. | Langmuir (2015) 31, 12187-12196 |
| 20-40 | mouse erythrocyte | vesicle | microfluidics | Jin, Verkman | Lab Chip (2015) 15, 3380-3390 |
2. Experimental Design, Materials and Methods
Preparation of Lipid Emulsions: DPhPC in chloroform (DphPC; 150 µL of 50 mg/mL: Avanti Polar Lipids, Alabaster, AL) was rotary evaporated to remove the solvent and was stored in a low-pressure environment of 17 hPa with a desiccator for six h or more [6,7]. It was then dissolved in 250 µL of hexadecane (50 mg/mL of hexadecane, Nacalai Tesque, Kyoto). For the cholesterol-containing emulsion, cholesterol (10 mg/mL) was mixed with 40 µg/mL DphPC solution in a 1:1 volume ratio.
Solutions: A 100-mM NaCl solution was used as a standard solution, and the osmolarity was measured using an osmometer (Osmometer 3250, Advanced Instruments, Inc., Norwood, MA) (165 mOsm/L). Different concentrations of urea (200, 400, 600, 800, and 100 mM) in 100 mM NaCl were prepared, and osmolarity was measured (362, 562, 758, 966, and 1164 mOsm/L, respectively).
Gramicidin A (Santa Cruz Biotechnology, Dallas, TX, USA) was dissolved in ethanol at 1 mmol/L ethanol solution as a stock solution [8]. It was further diluted in an aqueous solution.
Surface treatment of the capillary: A glass capillary (Borosilicate Glass Capillaries, TW150-4, World Precision Instruments, Inc., Sarasota, FL) with an inner diameter of 1.1 mm length of 50 mm was used. First, the capillary's inner surface was made hydrophobic to accommodate the interfacial monolayer. The surface was coated with SIGMACOTE (Sigma – Aldrich, St. Louis, MO) and stored for at least six h in a desiccator at 17 hPa. The hydrophobic surface was further coated with DphPC (40 mg/mL hexane) and then stored in a desiccator at 17 hPa for more than three h.
Glass capillary and syringe for drawing oil: Glass capillary filled with electrolytes and oil are shown (Fig. 3). The intercalated oil phase was aspirated using a syringe with a long fine tube of polymicrocapillary (200 µm in diameter, Polymicro Technologies, Inc., Phoenix, AX).
Fig. 3.
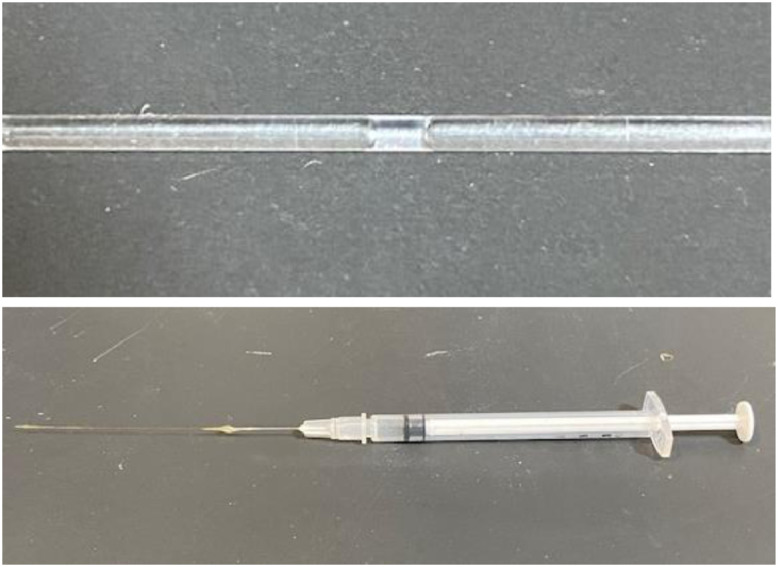
The capillary and the syringe for aspirating the oil. The capillary was filled with electrolyte and oil successively. The bulky oil phase was aspirated with the syringe with polymicrocapillary glued at the tip of the needle.
Electrode setting and electrophysiological measurements: Electrical measurements were performed for evaluating membrane capacitance and currents through the membrane-incorporated gramicidin A channel [9]. AgCl ink (BAS, 011464, Tokyo) was painted at one end for the capillary's inner surface [1]. For the other open end of the capillary, KCl-bridged Ag/AgCl electrodes were inserted into the electrolyte solution.
The membrane capacitance was measured by applying the ramp potential (±10 mV/ms). Given the specific membrane capacitance [1], the membrane area was estimated.
Image analysis for the membrane location and contact angle: The tangential view of the membrane (Fig. 4, left) was used to identify the membrane edge and evaluate the contact angle. In earlier work, the shape of the torus around the bilayer was theoretically solved [10]. Here, the points at the monolayers contacting the bilayer and the contact angle were evaluated through functional fitting to the torus contour [6,11]. First, bilayer and monolayer outlines in the tangential image were extracted using image analysis software (ImageJ, U. S. National Institutes of Health, Bethesda, MD) (Fig. 4 right). Next, the outline of the monolayer region of either side of the bilayer was fitted to an ellipse (green curves) using data analysis software (Origin Pro; OriginLab, Northampton, MA, USA). Then, the bilayer diameter was determined from a distance between the intersection points of both ellipses. The slope of tangent lines at intersection points was calculated, which is the contact angle of each side, and they were averaged.
Fig. 4.
The image of the bilayer and the torus and its contour with fitted lines (×400 magnification). For the bilayer area and the contact angle, the confluent points of the monolayers with the bilayer must be identified. Here, the contour of the image was fitted with an ellipse function (green lines).
Bilayer tension measurements: The method for evaluating the bilayer tension was described in the previous paper [6]. The monolayer tension was evaluated by a conventional method using the Young-Lippmann principle [6,12,13],
| (5) |
where Cbi is the bilayer capacitance, Vm is the membrane potential, and θ0 and θVm are the contact angles at 0 mV and Vm mV, respectively. Step voltages (+50 to +200 mV with an increment of 50 mV) were applied successively; each time after relaxation, the contact angle between the monolayer in the membrane torus and the bilayer was measured (Fig. 4). Then, from the contact angle, the bilayer tension was evaluated using the Young principle:
| (6) |
2.1. Streaming potential measurements for the gramicidin A channel
The use of the gA channel as a probe for the unstirred layer requires additional considerations. Under an osmotic gradient across the membrane, water flows through the gA channel. This flow generates the streaming potential by carrying cations through the pore under a single-file regime [14,15] even in the absence of an electrochemical potential gradient (Fig. 1).
Potassium activity (100 mM KCl) rather than concentration was set as identical in the solutions with and without the osmolyte using a potassium-selective electrode (model 9719, 720Aplus, Thermo Orion, Inc. Beverly, MA) [14]. The Vstream value was evaluated using the zero-current clamp mode (Fig. 1).
For the I-V curve measurements for the gramicidin channel, the streaming potential was corrected by adjusting the offset potential as zero mV under the current-clamp mode. By setting the offset potential, the net current through gA channels is nulled at 0 mV potential. The water flux through gA channels remains when the pore is empty of ions.
3. Experimental Setup
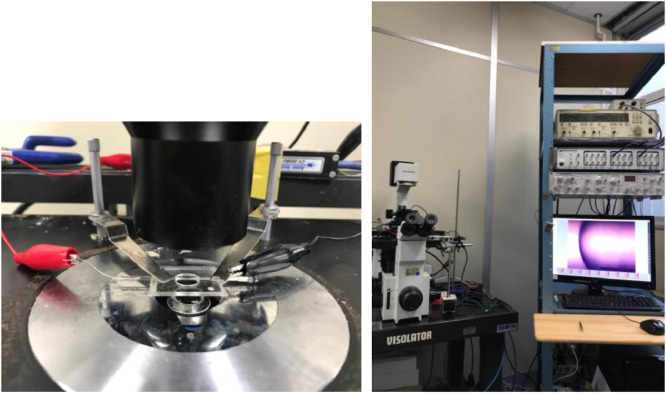
Photos of the experimental setup are shown (Fig. 5). A glass capillary set on the microscope stage is shown with the electrical connection (Fig. 5 left). The microscope is set on an anti-vibration Table 3. The electrophysiological setup, involving a patch-clamp amplifier, an analog-digital – digital-analog converter, and display for image analyses, is shown (Fig. 5 right).
Fig. 5.
The experimental setup. Left) The capillary set on the microscope stage, connected to the amplifier via the electrode. Right) the electrophysiological set with a camera for image analysis.
CRediT Author Statement
Keita Yano: Data curation, Writing- Original draft preparation. Masayuki Iwamoto: Conceptualization, Methodology, Image analysis. Takaaki Koshiji: Supervision. Shigetoshi Oiki: Conceptualization, Methodology, Writing- Reviewing and Editing.
Funding
This work was supported in part by KAKENHI Grants 20H03219 to M.I., and 19K22382 and 20H00497 to S.O.
Ethical Statement
Not applicable.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships which have or could be perceived to have influenced the work reported in this article.
Acknowledgments
We thank Drs. Hidehiko Okazawa (University of Fukui) for discussions.
References
- 1.Yano K., Iwamoto M., Koshiji T., Oiki S. Visualizing the osmotic water permeability of a lipid bilayer under measured bilayer tension using a moving membrane method. J. Membr. Sci. 2021;627 doi: 10.1016/j.memsci.2021.119231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levitt D.G., Elias S.R., Hautman J.M. Number of water molecules coupled to the transport of sodium, potassium and hydrogen ions via gramicidin, nonactin or valinomycin. BBA - Biomembr. 1978;512:436–451. doi: 10.1016/0005-2736(78)90266-3. [DOI] [PubMed] [Google Scholar]
- 3.Tripathi S., Hladky S.B. Streaming potentials in gramicidin channels measured with ion-selective microelectrodes. Biophys. J. 1998;74:2912–2917. doi: 10.1016/S0006-3495(98)77998-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenberg P.A., Finkelstein A. Interaction of ions and water in gramicidin A channels: streaming potentials across lipid bilayer membranes. J. Gen. Physiol. 1978;72:327–340. doi: 10.1085/jgp.72.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang K.W., Tripathi S., Hladky S.B. Ion binding constants for gramicidin A obtained from water permeability measurements. J. Membr. Biol. 1995;143:247–257. doi: 10.1007/BF00233453. [DOI] [PubMed] [Google Scholar]
- 6.Iwamoto M., Oiki S. Constitutive boost of a K+ channel via inherent bilayer tension and a unique tension-dependent modality. PNAS. 2018;115:13117–13122. doi: 10.1073/pnas.1812282115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iwamoto M., Shimizu H., Inoue F., Konno T., Sasaki Y.C., Oiki S. Surface structure and its dynamic rearrangements of the KcsA potassium channel upon gating and tetrabutylammonium blocking. J. Biol. Chem. 2006;281:28379–28386. doi: 10.1074/jbc.M602018200. M602018200 [pii] [DOI] [PubMed] [Google Scholar]
- 8.Oiki S., Koeppe R.E., Andersen O.S. Voltage-dependent gating of an asymmetric gramicidin channel. PNAS. 1995;92:2121–2125. doi: 10.1073/pnas.92.6.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oiki S., Koeppe R.E., Andersen O.S. Asymmetric gramicidin channels: heterodimeric channels with a single F6Val1 residue. Biophys. J. 1994;66:1823–1832. doi: 10.1016/S0006-3495(94)80976-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.White S.H. Analysis of the torus surrounding planar lipid bilayer membranes. Biophys. J. 1972;12:432–445. doi: 10.1016/S0006-3495(72)86095-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iwamoto M., Oiki S. Hysteresis of a tension-sensitive K+ Channel revealed by time-lapse tension measurements. JACS Au. 2021;1:467–474. doi: 10.1021/jacsau.0c00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taylor G.J., Venkatesan G.A., Collier C.P., Sarles S.A. Direct in situ measurement of specific capacitance, monolayer tension, and bilayer tension in a droplet interface bilayer. Soft Matter. 2015;11:7592–7605. doi: 10.1039/c5sm01005e. [DOI] [PubMed] [Google Scholar]
- 13.Oiki S., Iwamoto M. Lipid bilayers manipulated through monolayer technologies for studies of channel-membrane interplay. Biol. Pharm. Bull. 2018;41:303–311. doi: 10.1248/bpb.b17-00708. [DOI] [PubMed] [Google Scholar]
- 14.Ando H., Kuno M., Shimizu H., Muramatsu I., Oiki S. Coupled K+-water flux through the HERG potassium channel measured by an osmotic pulse method. J. Gen. Physiol. 2005;126:529–538. doi: 10.1085/jgp.200509377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iwamoto M., Oiki S. Counting ion and water molecules in a streaming file through the open-filter structure of the K channel. J. Neurosci. 2011;31:12180–12188. doi: 10.1523/JNEUROSCI.1377-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]