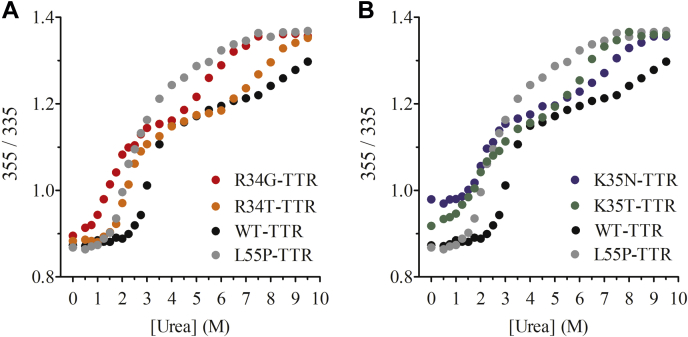
Figure 2.
Urea equilibrium denaturation of TTR disease associated variants.1.5 μM of WT- and pathogenic TTR variants were incubated for 96 h at 25 °C in 50 mM sodium phosphate (pH 7.4) containing 0.1 M KCl with increasing urea concentrations prior measurements of tryptophan fluorescence emission spectra. The tryptophan fluorescence emission intensity ratio displayed is defined as the ratio of the tryptophan emission intensity at 355 nm (unfolded state) to the tryptophan emission intensity at 335 nm (folded state) and is used as a measure of foldedness as was previously described (22). The missense variants in residues 34 (A) and 35 (B) are plotted in the same graph with the denaturation curves of WT-TTR and L55P TTR to compare the denaturation mechanism.