FIG 7.
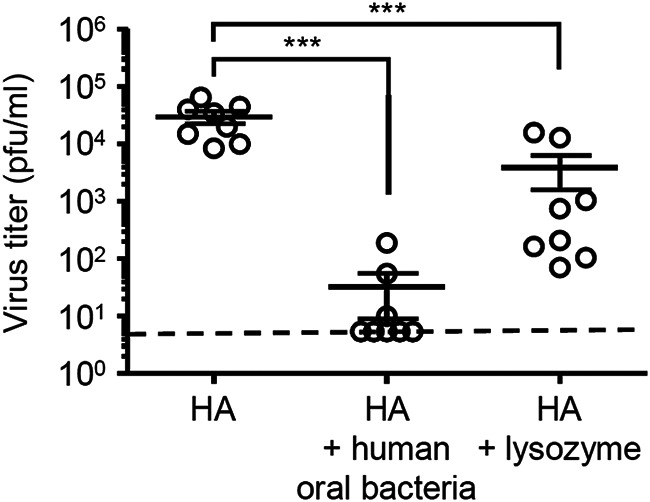
Protective effects of oral bacteria-adjuvanted intranasal vaccine against influenza virus infection. Mice were immunized intranasally with a quadrivalent HA vaccine with or without cultured oral bacteria from a healthy volunteer or lysozyme twice in a 3-week interval. Two weeks after the last vaccination, mice were challenged with 1,000 PFU of A/Narita/1/09 (pdm09). The nasal wash of influenza virus-infected mice was collected at 3 days postinfection, and viral titers were determined by plaque assay. Open circles indicate values for individual mice. The dashed line indicates the limit of virus detection. The data are from two independent experiments (mean ± SEM). ***, P < 0.001 (one-way ANOVA and Tukey’s test).
