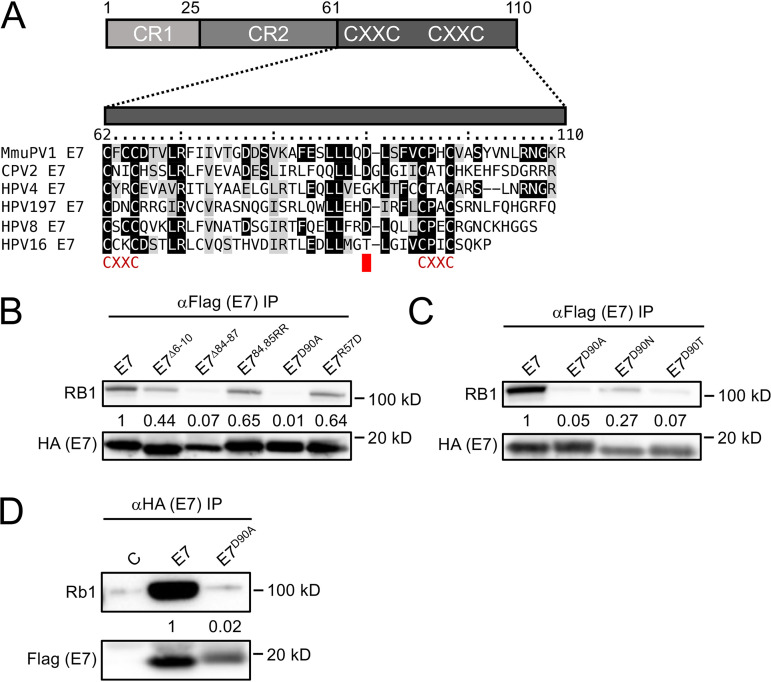
FIG 7.
The RB1 binding site maps to the MmuPV1 E7 C terminus. Sequence alignment of the C-terminal domains of MmuPV1, canine papillomavirus 2 (CPV2), γ1-HPV4, γ24-HPV197, β1-HPV8, and α9-HPV16 E7. Identical residues are marked by black boxes, and chemically similar residues are shaded in gray. The position of the CXXC motifs that form a zinc-binding site is shown. (A) The position of the aspartate residue at position 90 (D90) that is important for RB1 binding is indicated by a red box. (B) Various FLAG/HA-tagged MmuPV1 E7 mutants were expressed in HCT116 cells, and coprecipitated RB1 was detected by immunoblotting. The result shown is representative of six independent experiments. (C) Immunoprecipitation Western blot analyses to assess RB1 binding by various MmuPV1 E7D90 mutants. The result shown is representative of two independent experiments. Immunoprecipitation/Western blot analysis documenting that MmuPV1 E7D90A is defective for binding to murine Rb1. (D) Wild-type MmuPV1 E7 and the D90A mutant were transiently expressed in NIH 3T3 cells and binding assessed by immunoprecipitation/Western blotting. The blot shown is representative of 2 independent experiments. Quantifications of E7-coprecipitated RB1 or Rb1 are normalized to the amount of E7 that is precipitated and are shown underneath.