Key Points
Question
Should the definition of chronic kidney disease (CKD) consider the physiological estimated glomerular filtration rate (eGFR) decline that is associated with aging?
Findings
In this cohort study of people determined to have CKD using a fixed eGFR threshold but not according to age-adapted eGFR criteria, 75% were 65 years or older and had eGFR of 45 to 59 mL/min/1.73 m2 with normal/mild albuminuria. In this latter group, the risks of kidney failure and death were of similar magnitudes to those of controls without CKD.
Meaning
The study findings suggest that the current CKD definition that does not consider age-related eGFR decline may inflate the burden of CKD by classifying many elderly people with normal kidney aging as having a disease.
Abstract
Importance
Using the same level of estimated glomerular filtration rate (eGFR) to define chronic kidney disease (CKD) regardless of patient age may classify many elderly people with a normal physiological age-related eGFR decline as having a disease.
Objective
To compare the outcomes associated with CKD as defined by a fixed vs an age-adapted eGFR threshold.
Design, Setting, and Participants
This population-based cohort study was conducted in Alberta, Canada and used linked administrative and laboratory data from adults with incident CKD from April 1, 2009, to March 31, 2017, defined by a sustained reduction in eGFR for longer than 3 months below a fixed or an age-adapted eGFR threshold. Non-CKD controls were defined as being 65 years or older with a sustained eGFR of 60 to 89 mL/min/1.73 m2 for longer than 3 months and normal/mild albuminuria. The follow-up ended on March 31, 2019. The data were analyzed from February to April 2020.
Exposures
A fixed eGFR threshold of 60 vs thresholds of 75, 60, and 45 mL/min/1.73 m2 for age younger than 40, 40 to 64, and 65 years or older, respectively.
Main Outcomes and Measures
Competing risks of kidney failure (kidney replacement initiation or sustained eGFR <15 mL/min/1.73 m2 for >3 months) and death without kidney failure.
Results
The fixed and age-adapted CKD cohorts included 127 132 (69 546 women [54.7%], 57 586 men [45.3%]) and 81 209 adults (44 582 women [54.9%], 36 627 men [45.1%]), respectively (537 vs 343 new cases per 100 000 person-years). The fixed-threshold cohort had lower risks of kidney failure (1.7% vs 3.0% at 5 years) and death (21.9% vs 25.4%) than the age-adapted cohort. A total of 53 906 adults were included in both cohorts. Of the individuals included in the fixed-threshold cohort only (n = 72 703), 54 342 (75%) were 65 years or older and had baseline eGFR of 45 to 59 mL/min/1.73 m2 with normal/mild albuminuria. The 5-year risks of kidney failure and death among these elderly people were similar to those of non-CKD controls, with a risk of kidney failure of 0.12% or less in both groups across all age categories and a risk of death at 69, 122, 279, and 935 times higher than the risk of kidney failure for 65 to 69, 70 to 74, 75 to 79, and 80 years or older, respectively.
Conclusions and Relevance
This cohort study of adults with CKD suggests that the current criteria for CKD that use the same eGFR threshold for all ages may result in overestimation of the CKD burden in an aging population, overdiagnosis, and unnecessary interventions in many elderly people who have age-related loss of eGFR.
This cohort study examines outcomes associated with chronic kidney disease as defined by a fixed vs an age-adapted estimated glomerular filtration rate threshold.
Introduction
Chronic kidney disease (CKD) is a worldwide public health challenge and results in substantial morbidity, mortality, and health care costs.1,2 In adults, CKD is diagnosed based on the presence of abnormalities of kidney structure or function (ie, abnormal albuminuria or estimated glomerular filtration rate [eGFR]) for more than 3 months, with implications for health.3 The development of a uniform definition of CKD has been a useful paradigm that serves as a foundation for targeted screening and more precise diagnosis of CKD, appropriate patient care, and health care resource planning. Values of eGFR that are less than the threshold of 60 mL/min/1.73 m2 indicate CKD because they reflect an average loss of at least 50% of the kidney function in a healthy young adult.3 Many large studies have found that, starting from this threshold, lower eGFR values are associated with a graded increase in the relative risks of adverse outcomes in adults, such as kidney failure, cardiovascular events, and mortality across all age categories.4
Although there is consensus that persistent presence of abnormal albuminuria indicates CKD, the appropriateness of using a single eGFR threshold to define CKD, regardless of albuminuria, has been debated.5,6,7,8 Because eGFR declines with advancing age,9 a definition based on a fixed eGFR threshold may lead to underdiagnosis in young individuals and overdiagnosis in elderly individuals, whose eGFR physiologically declines with aging.10 A fixed-threshold definition may result in overestimation of the CKD burden in an aging population, largely attributable to people who may not have increased risks of adverse outcomes. An age-adapted CKD definition has been proposed, with eGFR thresholds of 75, 60, and 45 mL/min/1.73 m2 for younger than 40, 40 to 64, and 65 years or older, respectively.10 The differences in size and outcomes of the CKD population identified using the 2 definitions are unknown. It is also unclear to what extent the outcomes of people who have CKD only according to the current fixed eGFR threshold definition may differ from those who do not have CKD.
We conducted a population-based cohort study to identify adults with incident CKD according to the fixed-threshold and age-adapted threshold definitions. We estimated incidence rates of CKD and risks of kidney failure and death. We also compared these risks in people 65 years or older with normal/mild albuminuria who had CKD according to only the fixed-threshold definition (index eGFR, 45-59 mL/min/1.73 m2) with a control group of individuals with similar characteristics who did not have CKD (eGFR, 60-89 mL/min/1.73 m2).
Methods
Study Design and Data Sources
We conducted a population-based cohort study using linked administrative and laboratory data from Alberta, Canada (eFigure 1 in the Supplement).11 We calculated eGFR as described previously12 using the Chronic Kidney Disease Epidemiology Collaboration equation, with serum creatinine values standardized to isotope dilution mass spectrometry–traceable methods.13 We used the mean value of eGFR when there were multiple measurements on the same day. We only used outpatient eGFR measurements to minimize the inclusion of people with episodes of acute kidney injury. The institutional ethics review boards at the Universities of Alberta and Calgary approved this study with a waiver of participant consent because of the retrospective study design and secondary use of routinely collected administrative data. We followed recommended reporting standards (Reporting of Studies Conducted Using Observational Routinely-Collected Data [RECORD]).14
Study Population
CKD Populations
We identified adults (aged ≥18 years) who had incident CKD, which was defined by a sustained reduction in eGFR for more than 90 days, using 2 definitions. We used a fixed eGFR threshold of 60 mL/min/1.73 m2 to define CKD according to current criteria.3 For the age-adapted definition, we used thresholds of 75, 60, and 45 mL/min/1.73 m2 for younger than 40, 40 to 64, and 65 years or older, respectively.10 We screened each individual’s consecutive series of 2 or more eGFR measurements, in which the first and last eGFR measurements were separated by more than 90 days and all possible intervening measurements were within 90 days. We selected the earliest series (qualifying period) in which all measurements met the eGFR threshold requirement for cohort entry. The date of the last eGFR measurement (index eGFR) in the qualifying period defined the index date (cohort entry date), which was between April 1, 2009, and March 31, 2017. We excluded those who had received any form of kidney replacement therapy (dialysis or kidney transplantation) or had had an outpatient eGFR of less than 15 mL/min/1.73 m2 on or before the index date.
Non-CKD Controls
To compare outcomes by eGFR in elderly people with normal/mild albuminuria, we created a non-CKD cohort using the same methods described previously, with a sustained eGFR of 60 to 89 mL/min/1.73 m2 for longer than 3 months (higher eGFR values are associated with increased mortality in older people).15 Additional criteria for inclusion in the control cohort were being 65 years or older and having normal/mild albuminuria at baseline. We excluded people who had previously met a fixed-threshold or an age-adapted threshold definition of CKD before they became eligible for cohort entry.
Outcomes and Follow-up
The primary outcome was the earlier of kidney failure or all-cause mortality (ie, death before kidney failure), which we treated as competing events. We defined kidney failure as the earlier of the initiations of kidney replacement therapy (chronic dialysis or kidney transplantation) or a sustained eGFR of less than 15 mL/min/1.73 m2 for more than 90 days. We followed participants from the date of cohort entry until the earliest of kidney failure, death, or censoring (emigration from the province, 5 years after cohort entry, or March 31, 2019).
Covariates
Baseline variables included age, sex, index eGFR, albuminuria, diabetes, and cardiovascular disease (presence of congestive heart failure, myocardial infarction, peripheral vascular disease, or stroke). We also considered age and eGFR at the beginning of the qualifying period to describe the cohorts, as these dictated the eGFR thresholds that were used for the age-adapted definition. Albuminuria was categorized as normal/mild, moderate, severe, or unmeasured based on the most recent outpatient values, with the following types of measurement in descending order of preference: albumin:creatinine ratio (<30, 30 to 300, or >300 mg/g), protein:creatinine ratio (<150, 150 to 500, or >500 mg/g), and urine dipstick protein (negative or trace, 1+, or ≥2+).3 We used validated algorithms to identify comorbidities.16
Statistical Analysis
We used data on population estimates from Statistics Canada17 to estimate the incidence of CKD according to the 2 definitions, which were stratified by fiscal year and age at baseline. For the 2 CKD cohorts and subgroups captured by either or both definitions, we described baseline characteristics and used the nonparametric Aalen-Johansen method to estimate the cumulative incidence functions (ie, absolute risks) of kidney failure and death.
To investigate the associations between levels of index eGFR and kidney failure and death in people 65 years or older with normal/mild albuminuria, we summarized absolute risks at 5 years (a timeframe of predictions commonly used in clinical practice) for eGFR categories of 15 to 29, 30 to 44, 45 to 59, and 60 to 89 mL/min/1.73 m2, which were stratified by 65 to 69, 70 to 74, 75 to 79, and 80 years or older. We also estimated 2 measures of relative risk using the Fine and Gray (subhazard ratios) and Cox regression models (hazard ratios; eMethods in the Supplement). We used Stata, version 16 (StataCorp), and R, version 4.0.3 (R Foundation), for all analyses. We performed all data analyses from February to April 2020. We used a 2-sided P value of ≤ .05 for statistical significance.
Results
Incidence of CKD
The fixed-threshold and age-adapted threshold cohorts included 127 132 and 81 209 adults with incident CKD, respectively (eFigure 2 in the Supplement). The fixed-threshold definition yielded a 60% higher incidence rate than the age-adapted definition (537 vs 343 new cases per 100 000 person-years). Rate differences were more pronounced in those older than 65 years (age 65-79 years: 2356 vs 714; ≥80 years: 3767 vs 2597 per 100 000 person-years). Conversely, the fixed-threshold definition yielded an 85% lower incidence rate in those younger than 40 years (14 vs 91 per 100 000 person-years; Figure 1).
Figure 1. Incidence of Chronic Kidney Disease (CKD).
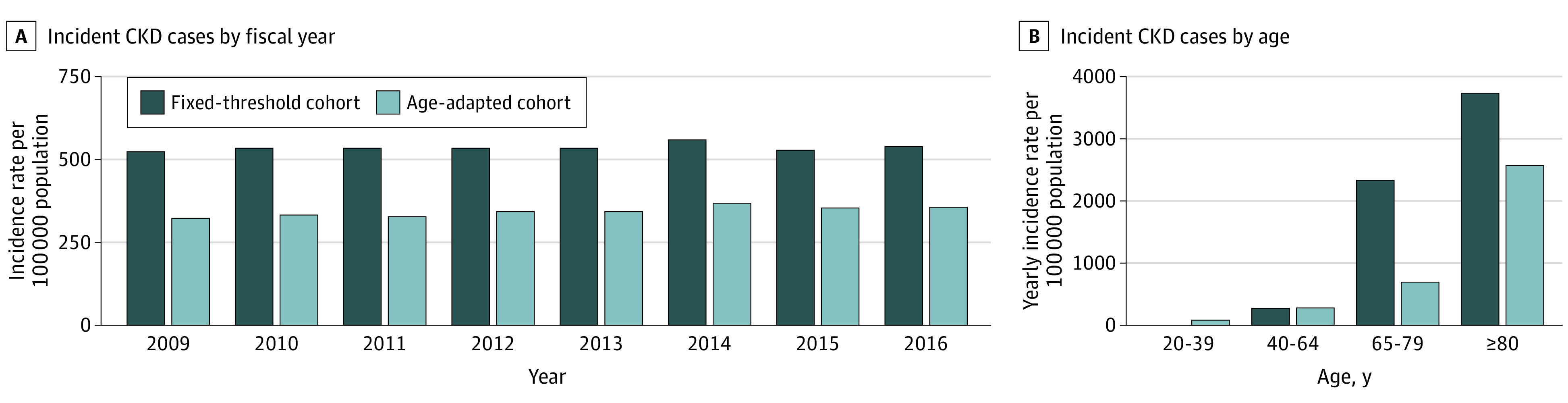
Incident CKD cases per 100 000 person-years identified using estimated glomerular filtration rate algorithms by fiscal years (A) and age (B) during the accrual period from April 1, 2009, to March 31, 2017.
Cohort Description
CKD Cohorts According to 2 eGFR Definitions
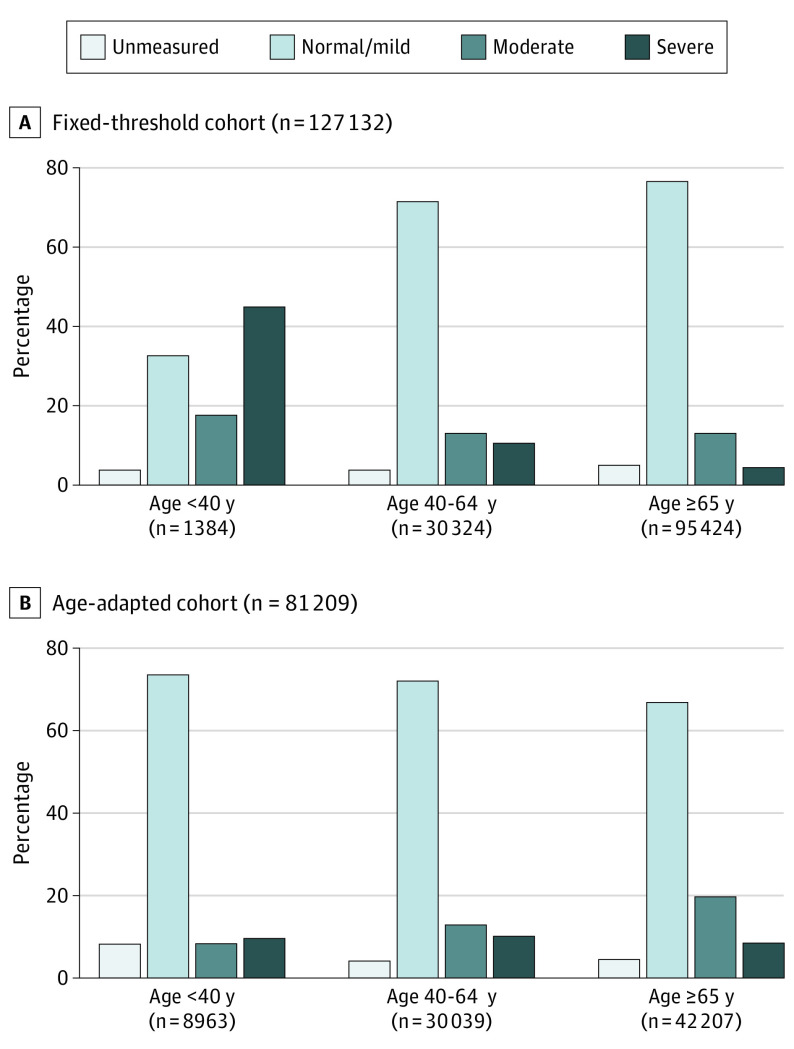
Compared with the age-adapted cohort, the fixed-threshold cohort was older, had a higher index eGFR, and was less likely to have moderate-to-severe albuminuria at cohort entry (20% vs 25%; Table). In the fixed-threshold cohort, older age groups were more likely to have normal/mild albuminuria (33%, 72%, and 77% for age <40, 40-64, and ≥65 years, respectively). The opposite was observed in the age-adapted cohort (74%, 72%, and 67%, respectively; Figure 2).
Table. Baseline Characteristics.
| Characteristics | No. (%) | |
|---|---|---|
| Fixed threshold (n = 127 132) | Age-adapted (n = 81 209) | |
| Female | 69 546 (54.7) | 44 582 (54.9) |
| Male | 57 586 (45.3) | 36 627 (45.1) |
| First age, median (range), ya | 72 (18-105) | 68 (18-109) |
| 18-39 | 1518 (1.2) | 9297 (11.4) |
| 40-64 | 33 346 (26.2) | 30 142 (37.1) |
| ≥65 | 92 268 (72.6) | 41 770 (51.4) |
| Index age, median (range), yb | 73 (18-107) | 68 (18-111) |
| 18-39 | 1384 (1.1) | 8963 (11.0) |
| 40-64 | 30 324 (23.9) | 30 039 (37.0) |
| ≥65 | 95 424 (75.1) | 42 207 (52.0) |
| First eGFR, median (range), mL/min/1.73 m2a | 54.9 (15.0-59) | 44.1 (15.0-74) |
| 60-74 | NA | 8000 (9.9) |
| 45-59 | 111 245 (87.5) | 27 267 (33.6) |
| 15-44 | 15 887 (12.5) | 45 942 (56.6) |
| Index eGFR, median (range), mL/min/1.73 m2b | 53.3 (15.0-59) | 43.3 (15.0-74) |
| 60-74 | NA | 7408 (9.1) |
| 45-59 | 103 749 (81.6) | 25 900 (31.9) |
| 15-44 | 23 383 (18.4) | 47 901 (59.0) |
| Albuminuriac | ||
| Unmeasured | 6503 (5.1) | 3983 (4.9) |
| Normal/mild | 95 602 (75.2) | 56 503 (69.6) |
| Moderate | 16 881 (13.3) | 13 042 (16.1) |
| Severe | 8146 (6.4) | 7681 (9.5) |
| CV and diabetesd | ||
| Neither | 62 947 (49.5) | 38 629 (47.6) |
| CV only | 25 025 (19.7) | 15 788 (19.4) |
| Diabetes only | 23 586 (18.6) | 14 492 (17.8) |
| Both | 15 574 (12.3) | 12 300 (15.1) |
Abbreviations: CV, cardiovascular disease, including congestive heart failure, myocardial infarction, peripheral vascular disease, or stroke; eGFR, estimated glomerular filtration rate; NA, not applicable.
First age and first eGFR refer to the values at the beginning of the qualifying period.
Last age (baseline age) and last eGFR (index eGFR) refer to the values at the end of the qualifying period (study entry or index date).
Albuminuria was categorized as normal or mild, moderate, severe, or unmeasured based on the most recent outpatient values before cohort entry, with the following types of measurement in descending order of preference: albumin-to-creatinine ratio (<30, 30 to 300, or >300 mg/g), protein-to-creatinine ratio (<150, 150 to 500, or >500 mg/g), and urine dipstick protein (negative or trace, 1+, or ≥2+).
Defined as the presence of CV disease or diabetes at baseline.
Figure 2. Baseline Albuminuria by Age.
Albuminuria was categorized as normal/mild, moderate, severe, or unmeasured based on the most recent outpatient values before cohort entry, with the following types of measurement in descending order of preference: albumin-to-creatinine ratio (<30, 30 to 300, or >300 mg/g), protein-to-creatinine ratio (<150, 150 to 500, or >500 mg/g), and urine dipstick protein (negative or trace, 1+, or ≥2+).
A total of 53 906 adults were included in both cohorts. Of these, 37 018 (69%) were captured by both definitions on the same day, 16 157 (30%) were captured a median of 1.9 years earlier by the fixed-threshold definition (interquartile range, 0.9-3.4 years), and 731 (1%) were captured a median of 1.4 years earlier (interquartile range, 0.5-2.8 years) by the age-adapted definition (eTables 1 and 2 in the Supplement).
A total of 72 703 of the fixed-threshold cohort members (57.2%) did not have CKD according to the age-adapted definition. A total of 54 342 (74.7%) of these people were 65 years or older and had an index eGFR of 45 to 59 mL/min/1.73 m2 with normal/mild albuminuria at baseline. A total of 30 093 (41.4%) of them had neither diabetes nor cardiovascular disease. People who met the age-adapted definition only were younger than 40 years at the beginning of the qualifying period (7817 [100%]). Most of them had normal/mild albuminuria (6291 [80.5%]; eTables 1-3 in the Supplement).
Non-CKD Elderly Controls
We identified 90 393 people aged 65 years or older who had a sustained eGFR of 60 to 89 mL/min/1.73 m2 for longer than 3 months with normal/mild albuminuria (eFigure 3 in the Supplement). Baseline characteristics are described in eTable 3 in the Supplement.
Outcome Analyses
Risks by eGFR-based CKD Definitions
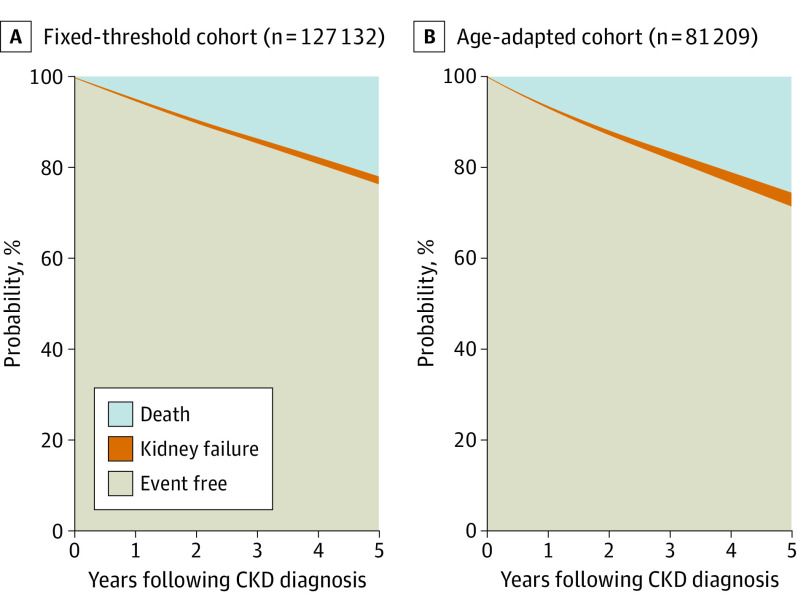
Risks of kidney failure and death at 5 years were 1.7% and 21.9%, respectively, in the fixed-threshold cohort and 3.0% and 25.4% in the age-adapted cohort (Figure 3). Risks of kidney failure and death at 5 years for subgroups of individuals captured by either or both definitions are shown in eFigure 4 in the Supplement.
Figure 3. Risks of Kidney Failure and Death From Time of Initial Chronic Kidney Disease (CKD) Diagnosis.
Areas represent risks, which were estimated using cumulative incidence functions over time. Risks of kidney failure at 5 years were 1.67% (95% CI, 1.60%-1.75%) for the fixed-threshold cohort and 3.01% (95% CI, 2.89%-3.14%) for the age-adapted cohort; risks of death at 5 years were 21.9% (95% CI, 21.6%-22.1%) and 25.4% (95% CI, 25.1%-25.7%), respectively.
Associations Between Index eGFR and Kidney Failure and Death in People 65 Years or Older With Normal/Mild Albuminuria
In people with an index eGFR of 15 to 44 mL/min/1.73 m2, lower eGFR was associated with higher absolute risks of adverse outcomes regardless of age (Figure 4). Most elderly people who entered the fixed-threshold cohort had an index eGFR of 45 to 59 mL/min/1.73 m2 (Figure 4). In these people, the 5-year absolute risk of kidney failure was similar in magnitude (≤0.12%) to the risk observed in people without CKD (eGFR, 60-89 mL/min/1.73 m2) across age categories. Their 5-year absolute risk of death was also comparable across age categories (eGFR, 45-59 vs 60-89 mL/min/1.73 m2: 8.3% vs 6.1% and 37.4% vs 40.8% for age 65-69 years and ≥80 years, respectively). Individuals whose index eGFR was 45 to 59 mL/min/1.73 m2 were increasingly more likely to die than to develop kidney failure with older age, from 69 times for individuals aged 65 to 69 years to 122, 279, and 935 times for age 70-74, 75-79, and 80 years or older, respectively (Figure 4). Compared with an eGFR of 60 to 89 mL/min/1.73 m2, an eGFR of 45-59 mL/min/1.73 m2 was associated with a statistically significant increase in the risk of kidney failure only in people aged 65 to 69 years and a statistically significant increase in the risk of death in those aged 65 to 74 years (eFigures 5 and 6 in the Supplement).
Figure 4. Risks of Kidney Failure (KF) and Death at 5 Years by Index Estimated Glomerular Filtration Rate (eGFR) and Age in Elderly People With Normal/Mild Albuminuria.
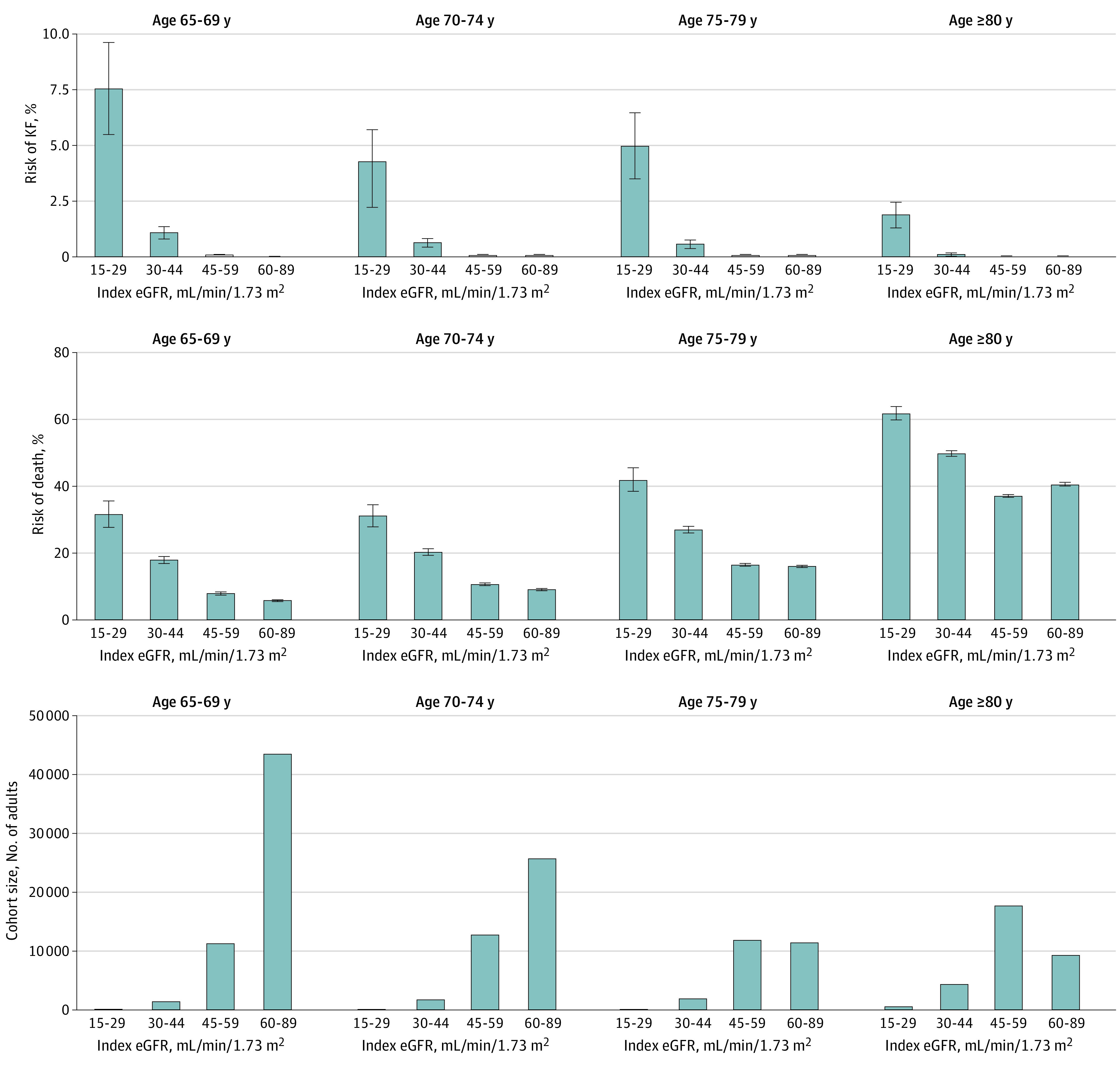
Analyses were restricted to people 65 years or older with normal/mild albuminuria. Chronic kidney disease (CKD) was defined using the fixed-threshold approach; individuals were assigned to eGFR categories based on the index eGFR when they first met the eGFR criteria for CKD. The absence of CKD was defined using a sustained eGFR of 60 to 89 mL/min/1.73 m2 for more than 3 months. C, Values for 15 to 29 index eGFR for ages 65 to 69, 70 to 74, and 75 to 70, respectively: 179, 226, and 227.
Discussion
According to this population-based study, the incidence, baseline characteristics, and outcomes of CKD vary substantially depending on whether the eGFR criteria for CKD consider age. First, using a single, fixed eGFR threshold irrespective of age resulted in a 60% higher incidence of CKD compared with an age-adapted definition and identified an older cohort with higher eGFR and lower albuminuria. Second, about 10% of people who met the age-adapted eGFR definition for CKD were not captured by the fixed eGFR definition. Although 5-year risks of kidney failure and death were low in these people, their young age (<40 years) warrants longer follow-up studies to better characterize their outcomes. Third, about 75% of people who had CKD only according to current eGFR criteria were 65 years or older and had an eGFR of 45 to 59 mL/min/1.73 m2 with normal/mild albuminuria. While the younger proportion of this large population (age <75 years) had increased relative risks of kidney failure and death compared with non-CKD controls, they were all far more likely to die than to develop kidney failure, and their absolute risks were similar in magnitude to those of non-CKD controls. These findings suggest the need for an age-adapted eGFR threshold to define CKD in people with normal/mild albuminuria.
We studied the predictive validity of each CKD definition as opposed to their current validity against histopathology data, which are difficult to obtain at a population level. Possible misclassification of kidney aging as kidney disease in elderly people with an eGFR of 45 to 59 mL/min/1.73 m2 and normal/mild albuminuria may explain a higher incidence rate of CKD in the fixed-threshold cohort compared with the age-adapted cohort. With advancing age, the kidneys undergo anatomical and physiological changes that result from normal senescence18 that unfold with variable speed from person to person but may not increase the risks of kidney failure and death.
Estimating CKD burden in a population is substantially affected by how CKD is defined. The overall incidence of CKD according to the fixed eGFR threshold definition was 60% higher than the age-adapted definition. While we found no relevant population data on the incidence of CKD, available prevalence data are consistent with our findings. A study from Iceland found similar excess in the estimated prevalence of CKD when adopting the fixed eGFR threshold definition compared with an age-adapted definition, with a population prevalence of CKD of 5.94% and 3.64%, respectively.19 Our study provides comparison data between the 2 definitions. First, people who had CKD according to current fixed eGFR criteria were more likely to be older, have a higher eGFR, and have normal albuminuria than those who had CKD according to an age-adapted definition. Second, the age-adapted definition may lead to earlier identification, evaluation, and treatment of young adults, providing earlier opportunities to prevent CKD progression. However, approximately one-third of people who met both eGFR-based definitions were identified 2 years later by the age-adapted definition. Risks following a 2-year delay in diagnosis increased from 1.4% to 2.7% at 5 years for kidney failure and 25.0% to 41.6% for death. While there are potential benefits associated with timely patient identification, referral for specialist care, and management of treatable disorders,20 how such interventions may make a difference remains unclear when the absolute risk of kidney failure is as low as those observed in this study (<3%). Instead, the excess mortality in this group suggests that the fixed-threshold approach may enable earlier identification of people who are at higher risk of death than kidney failure. Finally, our study adds novel information on absolute risks of kidney failure and death in elderly individuals. Consistent with CKD Prognosis Consortium data, we found that an eGFR of 45 to 59 mL/min/1.73 m2 is independently associated with a higher relative risk of death up to age 75 years.15 Prior studies have documented a decrease in the hazard ratios of kidney failure and an increase in the hazard ratios of death with older age.21,22 To our knowledge, no previous studies have investigated absolute risks. We found that in elderly individuals (≥65 years) with an eGFR of 45-59 mL/min/1.73 m2 and normal/mild albuminuria, the absolute risk of death is many-fold higher than the risk of kidney failure, and the risks of kidney failure and death were similar in magnitude to those observed in people without CKD.
Our study has implications for clinical practice, health policy, and research. Optimal use of either eGFR-based CKD definition should consider their merits and drawbacks. Although the cutoffs we used reflect existing CKD staging, they are arbitrary. Applying an age-adapted definition will result in earlier identification of young people at increased risk of kidney failure and minimize the inclusion of elderly people with age-related loss of eGFR. However, age calibration adds a layer of complexity to CKD definition using arbitrary age thresholds and may lead to disease definition based on age without a corresponding change in eGFR.7 Applying the fixed-threshold CKD definition to an aging population will result in higher estimates of CKD burden in the population, with implications for planning and allocating health services and resources.23 More than half of individuals labeled as having CKD using current eGFR criteria are elderly people with similar risks of adverse outcomes as those without CKD. Labeling them with CKD may lead to unnecessary kidney-related interventions that minimally affect kidney outcomes, such as excessive referrals to nephrology care, more frequent nephrology laboratory testing, or diagnostic procedures.8,10,24,25,26 Instead, these elderly people may benefit from identification and evidence-based interventions for coexisting conditions (such as cardiovascular disease) or other modifiable risk factors associated with mortality.
Strengths and Limitations
This study has several strengths, including its population-based design in a geographically defined population with universal access to health care (including serum creatinine assessment and kidney replacement therapy). Furthermore, we applied a longer than 3 months duration criterion and used only outpatient eGFR measurements to minimize the inclusion of people with episodes of acute kidney injury when forming study cohorts. We also used a long washout period to maximize the inclusion of incident CKD cases and non-CKD controls. Consistent with current CKD staging criteria, we considered initiation of kidney replacement therapy and eGFR criteria to define kidney failure. Finally, we compared 2 definitions that use different eGFR criteria and included analyses restricted to elderly people with normal/mild albuminuria to study the ability of eGFR thresholds to define CKD, acknowledging that CKD is not questioned in the presence of abnormal albuminuria.
Our study also has limitations. First, the study source population was predominantly White, and this may limit the generalizability of our findings to populations with different racial and ethnic composition. Second, the study population was based on current guideline recommendations for eGFR calculation.3 Our findings may not apply when other methods are used to measure or estimate GFR. Third, the duration of our study may have been insufficient for kidney failure and mortality outcomes in younger individuals captured by the age-adapted definition only. Longer follow-up studies are needed to compare outcomes of this smaller population with those who do not have CKD according to the fixed-threshold definition. Lastly, our analysis focused on mortality and kidney failure. We did not examine quality of life, multimorbidity, polypharmacy, frailty, risk of acute kidney injury, and other outcomes that may be important for older individuals with CKD. Prospective studies are needed to clarify the extent to which any possible association may be the result of aging or the eGFR decline that accompanies aging.
Conclusions
This population-based study suggests that current eGFR criteria for CKD that do not account for the age-related eGFR decline may result in overestimation of the CKD burden in an aging population. The excess incidence of CKD with current eGFR criteria is largely explained by elderly people who have an eGFR of 45 to 59 mL/min/1.73 m2 and normal/mild albuminuria. These people have a much higher absolute risk of death than kidney failure, and their risks of kidney failure and death are similar in magnitude to people of the same age who do not have CKD. These risk profiles suggest that an eGFR of 45 to 59 mL/min/1.73 m2 without albuminuria in elderly individuals may deserve recognition and management strategies to target modifiable risk factors for death but may not have implications for kidney health.
eMethods.
eTable 1. Baseline characteristics, captured in the fixed-threshold CKD cohort
eTable 2. Baseline characteristics, captured in the age-adapted CKD cohort
eTable 3. Baseline characteristics of people aged ≥65 years with normal/mild albuminuria, by index eGFR
eFigure 1. Study design and data sources
eFigure 2. Cohort formation for two eGFR-based CKD cohorts
eFigure 3. Cohort formation for non-CKD controls
eFigure 4. Risks of kidney failure and death at 5 years, in subgroups defined by the agreement between the fixed and age-adapted eGFR threshold for CKD
eFigure 5. Sub-distribution hazard model
eFigure 6. Hazard model
References
- 1.Gansevoort RT, Matsushita K, van der Velde M, et al. ; Chronic Kidney Disease Prognosis Consortium . Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes: a collaborative meta-analysis of general and high-risk population cohorts. Kidney Int. 2011;80(1):93-104. doi: 10.1038/ki.2010.531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matsushita K, van der Velde M, Astor BC, et al. ; Chronic Kidney Disease Prognosis Consortium . Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375(9731):2073-2081. doi: 10.1016/S0140-6736(10)60674-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group . KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3(1):112-119. https://kdigo.org/wp-content/uploads/2017/02/KDIGO_2012_CKD_GL.pdf [Google Scholar]
- 4.Levey AS, de Jong PE, Coresh J, et al. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int. 2011;80(1):17-28. doi: 10.1038/ki.2010.483 [DOI] [PubMed] [Google Scholar]
- 5.Levey AS, Inker LA, Coresh J. Chronic kidney disease in older people. JAMA. 2015;314(6):557-558. doi: 10.1001/jama.2015.6753 [DOI] [PubMed] [Google Scholar]
- 6.Glassock R, Delanaye P, El Nahas M. An age-calibrated classification of chronic kidney disease. JAMA. 2015;314(6):559-560. doi: 10.1001/jama.2015.6731 [DOI] [PubMed] [Google Scholar]
- 7.Levey AS, Inker LA, Coresh J. “Should the definition of CKD be changed to include age-adapted GFR criteria?”: con: the evaluation and management of CKD, not the definition, should be age-adapted. Kidney Int. 2020;97(1):37-40. doi: 10.1016/j.kint.2019.08.032 [DOI] [PubMed] [Google Scholar]
- 8.Glassock RJ, Delanaye P, Rule AD. Should the definition of CKD be changed to include age-adapted GFR criteria? YES. Kidney Int. 2020;97(1):34-37. doi: 10.1016/j.kint.2019.08.033 [DOI] [PubMed] [Google Scholar]
- 9.Denic A, Glassock RJ, Rule AD. Structural and functional changes with the aging kidney. Adv Chronic Kidney Dis. 2016;23(1):19-28. doi: 10.1053/j.ackd.2015.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delanaye P, Jager KJ, Bökenkamp A, et al. CKD: a call for an age-adapted definition. J Am Soc Nephrol. 2019;30(10):1785-1805. doi: 10.1681/ASN.2019030238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hemmelgarn BR, Clement F, Manns BJ, et al. Overview of the Alberta Kidney Disease Network. BMC Nephrol. 2009;10:30. doi: 10.1186/1471-2369-10-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ravani P, Fiocco M, Liu P, et al. Influence of mortality on estimating the risk of kidney failure in people with stage 4 CKD. J Am Soc Nephrol. 2019;30(11):2219-2227. doi: 10.1681/ASN.2019060640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levey AS, Stevens LA, Schmid CH, et al. ; Chronic Kidney Disease Epidemiology Collaboration . A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604-612. doi: 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benchimol EI, Smeeth L, Guttmann A, et al. ; RECORD Working Committee . The Reporting of Studies Conducted Using Observational Routinely-collected Health Data (RECORD) statement. PLoS Med. 2015;12(10):e1001885. doi: 10.1371/journal.pmed.1001885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tonelli M, Wiebe N, Fortin M, et al. ; Alberta Kidney Disease Network . Methods for identifying 30 chronic conditions: application to administrative data. BMC Med Inform Decis Mak. 2015;15:31. doi: 10.1186/s12911-015-0155-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Statistics Canada . Table 17-10-0005-01 Population estimates on July 1st, by age and sex. Accessed October 11, 2020. https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1710000501
- 17.Glassock RJ, Rule AD. Aging and the kidneys: anatomy, physiology and consequences for defining chronic kidney disease. Nephron. 2016;134(1):25-29. doi: 10.1159/000445450 [DOI] [PubMed] [Google Scholar]
- 18.Jonsson AJ, Lund SH, Eriksen BO, Palsson R, Indridason OS. The prevalence of chronic kidney disease in Iceland according to KDIGO criteria and age-adapted estimated glomerular filtration rate thresholds. Kidney Int. 2020;98(5):1286-1295. doi: 10.1016/j.kint.2020.06.017 [DOI] [PubMed] [Google Scholar]
- 19.Carroll JK, Pulver G, Dickinson LM, et al. Effect of 2 clinical decision support strategies on chronic kidney disease outcomes in primary care: a cluster randomized trial. JAMA Netw Open. 2018;1(6):e183377. doi: 10.1001/jamanetworkopen.2018.3377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hallan SI, Matsushita K, Sang Y, et al. ; Chronic Kidney Disease Prognosis Consortium . Age and association of kidney measures with mortality and end-stage renal disease. JAMA. 2012;308(22):2349-2360. doi: 10.1001/jama.2012.16817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Hare AM, Choi AI, Bertenthal D, et al. Age affects outcomes in chronic kidney disease. J Am Soc Nephrol. 2007;18(10):2758-2765. doi: 10.1681/ASN.2007040422 [DOI] [PubMed] [Google Scholar]
- 22.Hemmelgarn BR, James MT, Manns BJ, et al. ; Alberta Kidney Disease Network . Rates of treated and untreated kidney failure in older vs younger adults. JAMA. 2012;307(23):2507-2515. doi: 10.1001/jama.2012.6455 [DOI] [PubMed] [Google Scholar]
- 23.Carter SM, Rogers W, Heath I, Degeling C, Doust J, Barratt A. The challenge of overdiagnosis begins with its definition. BMJ. 2015;350:h869. doi: 10.1136/bmj.h869 [DOI] [PubMed] [Google Scholar]
- 24.Moynihan R, Glassock R, Doust J. Chronic kidney disease controversy: how expanding definitions are unnecessarily labelling many people as diseased. BMJ. 2013;347:f4298. doi: 10.1136/bmj.f4298 [DOI] [PubMed] [Google Scholar]
- 25.Stevens RJ, Evans J, Oke J, et al. Kidney age, not kidney disease. CMAJ. 2018;190(13):E389-E393. doi: 10.1503/cmaj.170674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ellam T, Twohig H, Khwaja A. Chronic kidney disease in elderly people: disease or disease label? BMJ. 2016;352:h6559. doi: 10.1136/bmj.h6559 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eTable 1. Baseline characteristics, captured in the fixed-threshold CKD cohort
eTable 2. Baseline characteristics, captured in the age-adapted CKD cohort
eTable 3. Baseline characteristics of people aged ≥65 years with normal/mild albuminuria, by index eGFR
eFigure 1. Study design and data sources
eFigure 2. Cohort formation for two eGFR-based CKD cohorts
eFigure 3. Cohort formation for non-CKD controls
eFigure 4. Risks of kidney failure and death at 5 years, in subgroups defined by the agreement between the fixed and age-adapted eGFR threshold for CKD
eFigure 5. Sub-distribution hazard model
eFigure 6. Hazard model