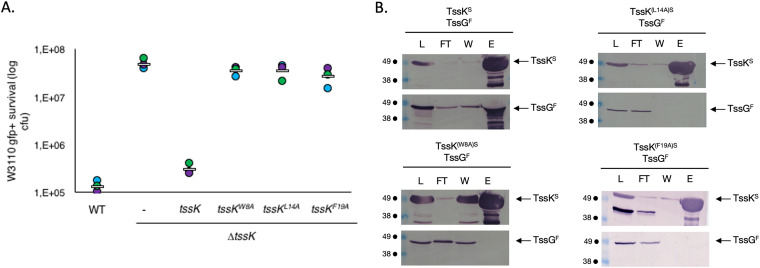
FIG 3.
TssG foot domains are essential for T6SS functioning. (A) Antibacterial assay. The relative fluorescent level (in arbitrary units) and the number of recovered Escherichia coli recipient cells (in log10 CFU) are indicated. The assays were performed at least three independent times, with technical triplicates, and the measurements of a representative technical triplicate (circles) are shown, with the corresponding average value calculated for all assays (white bar). (B) Interaction between Strep-tagged TssK (TssKS) and Flag-tagged TssG (TssGF) studied by affinity copurification. Copurification was carried between TssGF and variants of TssKS. TssK harboring the mutations W8A, L14A, and F19A are indicated by TssK(W8A)S, TssK(L14A)S, and TssK(F19A)S, respectively. Soluble extracts of E. coli BL21(DE3) cells producing the proteins indicated on top of the gels were submitted to an affinity purification step on a StrepTrap column, pulling down Strep-tagged TssK. The lysate (total soluble material [L]), flowthrough (FT), wash (W), and eluate (E) were subjected to denaturation by 12.5% acrylamide PAGE and subsequently immunodetected with the appropriate antibody. Immunodetected proteins are indicated on the right, while molecular weight markers (in kDa) are indicated on the left. As observed for wild-type TssKS, the simultaneous presence of a band in the eluate of TssK and a band in the eluate of TssG indicates the presence of interaction between the two proteins. As observed for all three TssKs mutants, the presence of a band in the eluate of TssK and the absence of a band in the eluate of TssG indicate the lack of interaction.