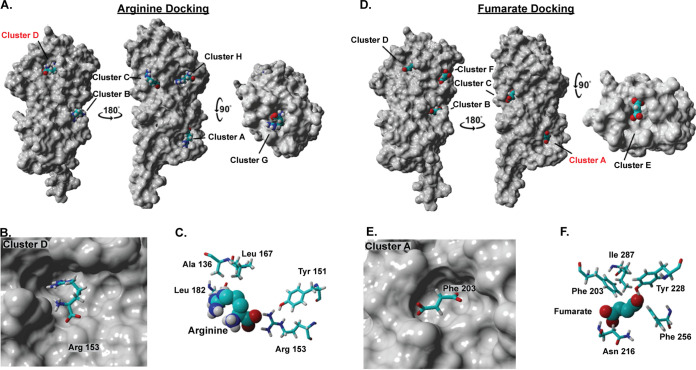
FIG 2.
Docking analysis of TlpA and arginine or fumarate identifies several clusters that are occupied by these ligands. (A and D) Docking analysis shows several clusters occupied by arginine (A) and fumarate (D) on the surface of a space-filling version of TlpALBD. Clusters C and G are biologically irrelevant due to the homodimer formation of TlpA, and clusters B and H are poorly populated (see Table S3 in the supplemental material). (B and C) View of the energetically preferred bound conformation of arginine in cluster D, the predicted membrane-distal dCache pocket, with key interacting amino acids shown. (E and F) View of the energetically preferred bound conformation of fumarate in cluster A, the membrane-proximal dCache pocket, with key interacting amino acids shown.