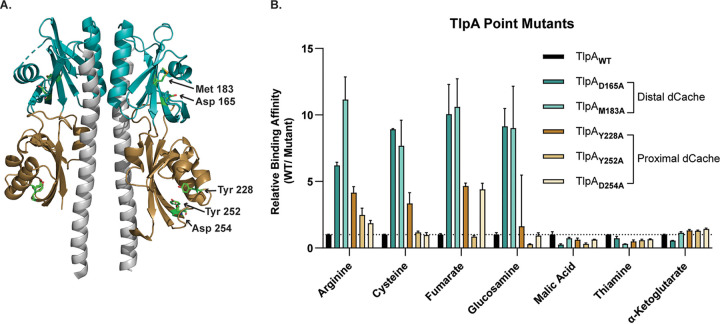
FIG 3.
TlpALBD binds chemotaxis-active ligands through the membrane-distal or -proximal dCache subdomains. (A) Ribbon diagram of TlpALBD as a homodimer. The membrane-distal and membrane-proximal dCache domains are shown in teal and gold, respectively. Residues that were mutated to alanine are highlighted in each region to make TlpAD165A, TlpAM183A, TlpAY228A, TlpAY252A, and TlpAD254A. (B) Relative binding affinities of each ligand for the WT and TlpALBD membrane-distal and -proximal dCache point mutants. For example, mutation of D165 to A resulted in an ∼6-fold decrease in the arginine binding affinity compared to WT binding. Data represent the mean values and standard errors of the means from three independent experiments (n = 3).