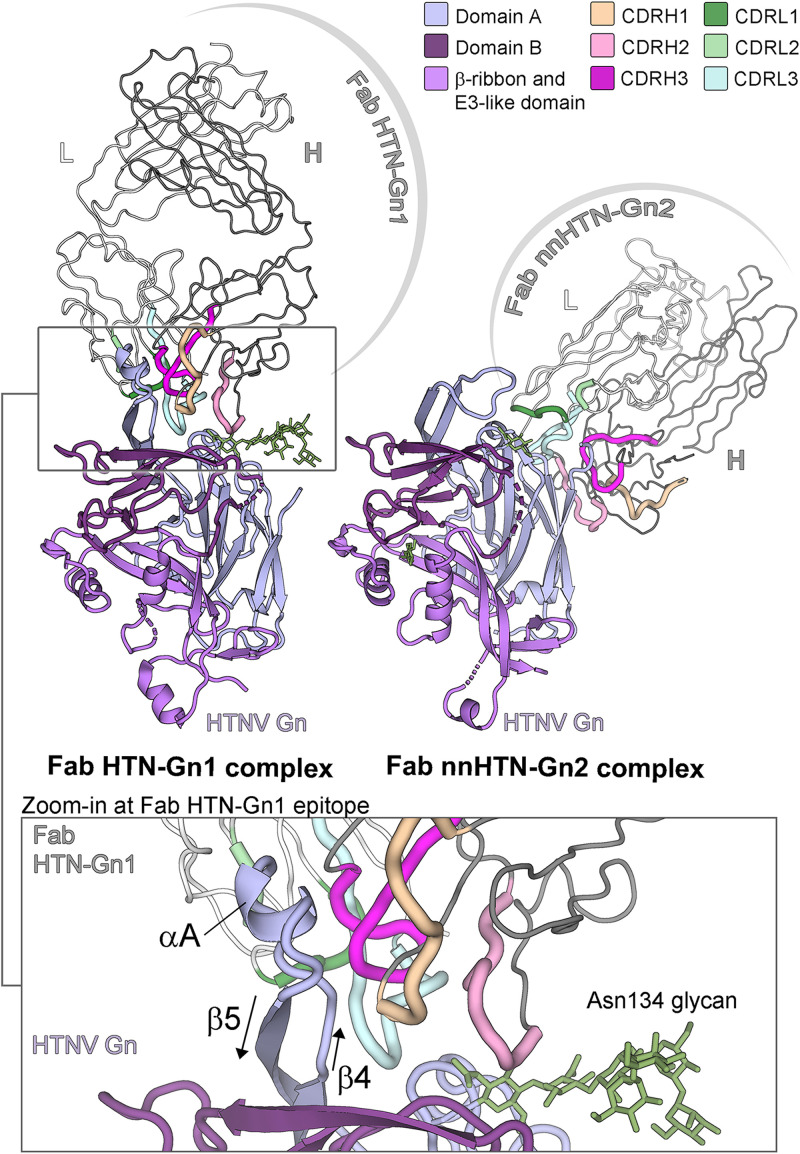
FIG 3.
Crystal structures of Fab fragments from neutralizing mAb HTN-Gn1 and non-neutralizing mAb nnHTN-Gn2 in complex with HTNV Gn reveal that the antibodies target disparate epitopes on the HTNV Gn surface. (Upper left) Structure of the HTNV Gn−HTN-Gn1 complex. The heavy and light chains of Fab HTN-Gn1 are colored dark and light gray, respectively. The CDR loops of the Fab are colored shades of pink (heavy chain) and green (light chain), respectively, as defined in the upper right legend. HTNV Gn is colored according to domain, with domain A in light blue, domain B in dark purple, and the β-ribbon/E3-like domain in purple, as defined in the upper right legend. (Upper right) Structure of the HTNV Gn−nnHTN-Gn2 complex. Colored as described for panel A. The Fab nnHTN-Gn2 binds to domain A of HTNV Gn in an interaction that relies on the CDRs of both heavy and light chains and occludes 900 Å2 of surface area. (Lower) Zoom-in of the HTNV Gn−HTN-Gn1 interface. The ordered glycan extending from Asn134 (green sticks) of HTNV Gn from the Fab HTN-Gn1 complex was likely protected from endoglycosidase F1 during sample preparation and is stabilized by neighboring crystal contacts. Detailed representations of the interactions at the antibody-antigen interfaces are provided in Fig. S3 and S4 in the supplemental material.