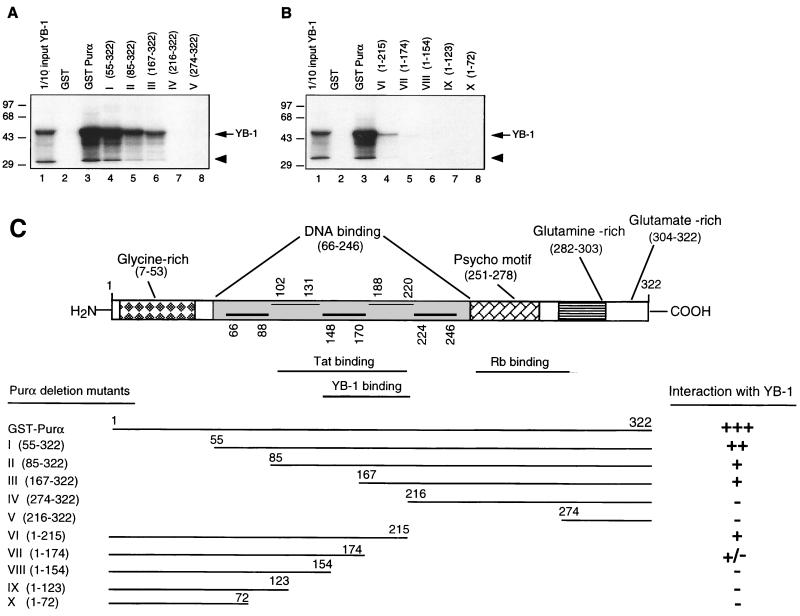
FIG. 6.
Mapping of the domain(s) of Purα involved in interaction with YB-1. (A and B) Mapping of the interaction domain of Purα with YB-1. GST-Purα or its N-terminal (A) or its C-terminal (B) deletion mutants were incubated with in vitro-translated [35S]methionine-labeled YB-1. Sepharose beads were washed three times with lysis buffer, and bound proteins were resolved by SDS–10% PAGE. One-tenth of the input YB-1 used in each reaction was loaded for migration controls (lane 1 in each panel). The labeled arrow marks the position of in vitro-translated [35S]methionine-labeled YB-1, and the arrowhead indicates a degradation product of YB-1. Numbers on the left are molecular masses in kilodaltons. (C) Summary of the results obtained from in vitro mapping assays. A schematic representation of the Purα protein is shown at the top (not shown to scale). The relative strengths of the interactions between GST-Purα and its deletion mutants with in vitro-translated [35S]methionine-labeled YB-1 are depicted.