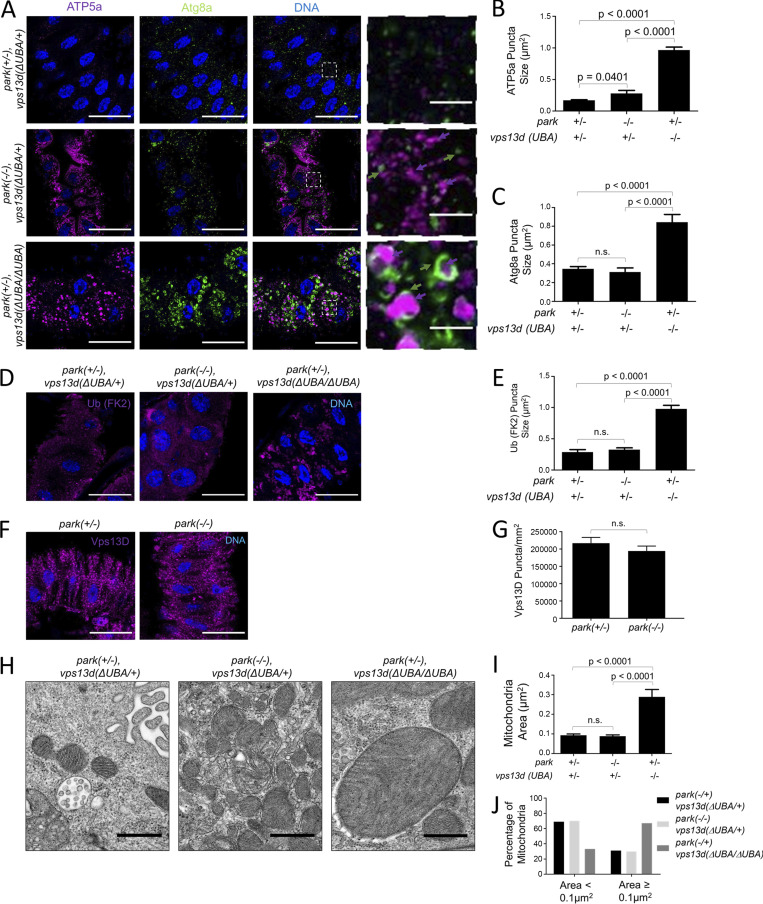
Figure 6.
Vps13D and Park function in separate mitochondrial clearance pathways. (A) park25/park25 (−/−);vps13d (ΔUBA/+) single-mutant intestine cells (middle panels) and park25/+ (+/−);vps13d (ΔUBA/ΔUBA) single-mutant intestine cells (bottom panels) 2 h after pupariation are unable to clear mitochondria as shown by ATP5a staining (purple) compared with park25/+ (+/−);vps13d (ΔUBA/+) heterozygous control cells (top panels). vps13d (ΔUBA) mutants have ATP5a that appear larger and less filamentous (purple arrows) and larger Atg8a puncta (green arrows) encircling the enlarged ATP5a puncta (purple arrows), which are not present in the park25 mutant cells. (B) Quantification of ATP5a puncta size in park25/+ (+/−);vps13d (ΔUBA/+) heterozygous control (n = 13), park25/park25 (−/−);vps13d (ΔUBA)/+ (n = 12), and park25/+ (+/−);vps13d (ΔUBA/ΔUBA) (n = 12) intestine cells. (C) Quantification of Atg8a puncta size in park25/+ (+/−);vps13d (ΔUBA/+) heterozygous control (n = 13), park25/park25 (−/−);vps13d (ΔUBA)/+ (n = 12), and park25/+ (+/−);vps13d (ΔUBA) (n = 12) intestine cells. (D) park25/+ (+/−);vps13d (ΔUBA/ΔUBA) single-mutant cells have larger conjugated ubiquitin (FK2) puncta (purple) than park25/park25 (−/−);vps13d (ΔUBA/+) single-mutant and park25/+ (+/−), vps13d (ΔUBA/+) heterozygous control intestine cells 2 h after pupariation. (E) Quantification of conjugated ubiquitin (FK2) size in park25/+ (+/−);vps13d (ΔUBA/+) heterozygous control (n = 11), park25/park25 (−/−);vps13d (ΔUBA)/+ (n = 12), and park25/+ (+/−);vps13d (ΔUBA/ΔUBA) (n = 11) intestine cells 2 h after pupariation. (F) park25/park25 (−/−) mutant intestine cells have similar levels of Vps13D puncta (purple) compared with heterozygous park25/+ (+/−) control intestine cells 2 h after pupariation. (G) Quantification of Vps13D puncta in park25/park25 (−/−) mutant intestine cells (n = 11) compared with park25/+ (+/−) control cells (n = 13). (H) Representative TEM images of park25/+ (+/−);vps13d (ΔUBA)/+ heterozygous control, park25/park25 (−/−);vps13d (ΔUBA/+) single-mutant, and park25/+ (+/−);vps13d (ΔUBA/ΔUBA) single-mutant intestine cells 2 h after pupariation. (I) Quantification of mitochondrial area from TEM images of park25/+ (+/−);vps13d (ΔUBA)/+ heterozygous control cells (n = 192), park25/park25 (−/−);vps13d (ΔUBA/+) single-mutant (n = 186), and park25/+ (+/−);vps13d (ΔUBA/ΔUBA) single-mutant (n = 184) intestine cells 2 h after pupariation. (J) Quantification of the percentage of mitochondria <0.1 μm and ≥0.1 μm from TEM images from park25/+ (+/−);vps13d (ΔUBA)/+ heterozygous control (n = 192), park25/park25 (−/−);vps13d (ΔUBA/+) single-mutant (n = 186), and park25/+ (+/−);vps13d (ΔUBA/ΔUBA) single-mutant (n = 184) intestine cells 2 h after pupariation. vps13d mutant intestine cells have larger mitochondria and fewer remaining mitochondria ≤0.1 μm2 than park mutant and control intestine cells. Columns were compared using Fisher’s exact test with the following values: park25/+;vps13d (ΔUBA)/+ vs. park25/park25;vps13d (ΔUBA/+), P = 0.9109, park25/+;vps13d (ΔUBA)/+ vs. park25/+;vps13d (ΔUBA/ΔUBA), P < 0.0001, and park25/park25;vps13d (ΔUBA/+) vs. park25/+;vps13d (ΔUBA/ΔUBA), P < 0.0001. Scale bars in A, D, and F are 40 μm with the exception of the enlarged images in A, which are 5 μm. Scale bars in H are 0.5 μm. Columns in B, C, E, and I were compared using one-way ANOVA with Tukey’s post hoc analysis. Columns in G were compared using two-tailed t test without Welch’s correlation. Error bars are SEM. Representative of three or more independent biological experiments.