Figure 6.
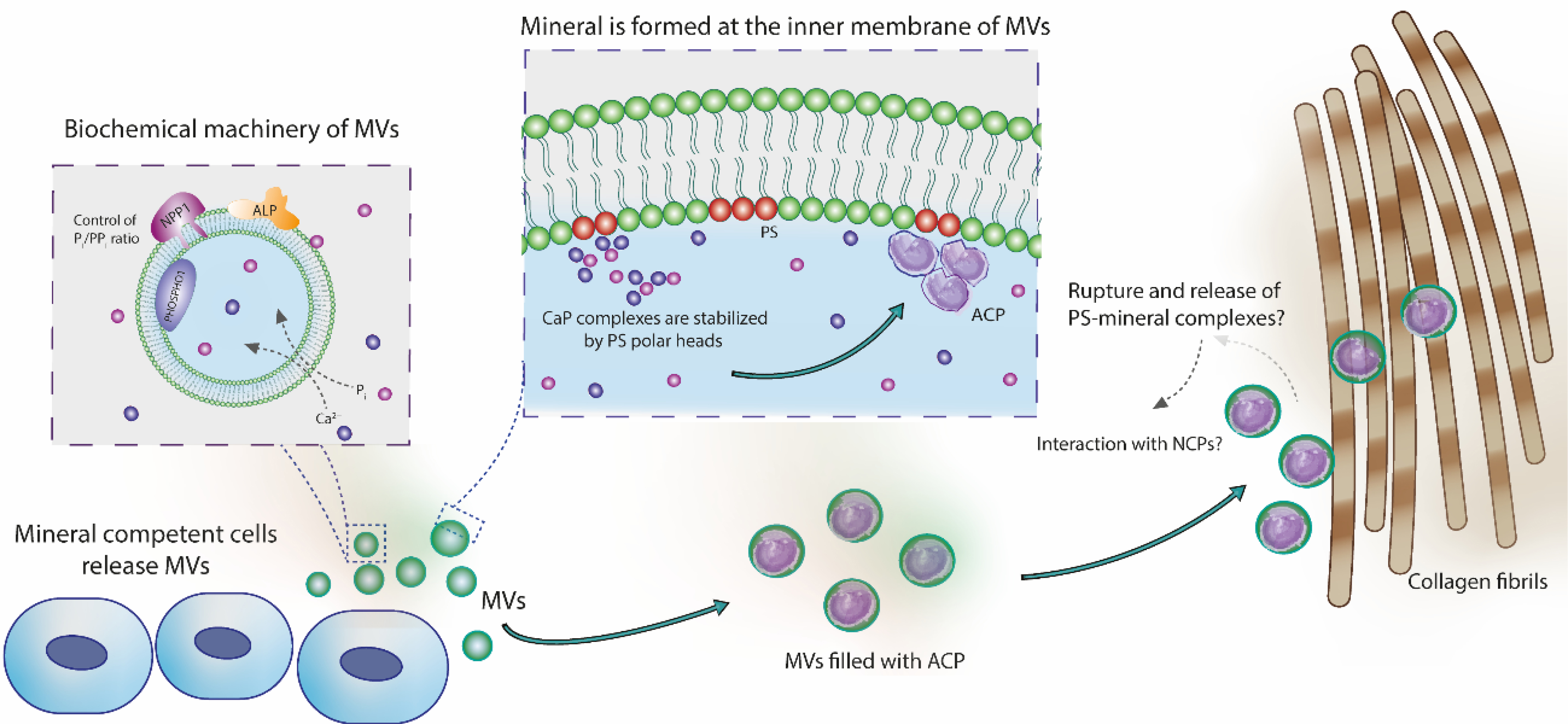
Role of MVs in the nucleation of calcium phosphates and in the mineralization of collagen fibrils. Mineral-competent cells release MVs harboring an enzymatic machinery capable of controlling the phosphate/pyrophosphate ratio required to trigger mineralization. These vesicles are rich on phosphatidylserine (PS) in their lumen, creating a highly negatively charged interface for nucleation and stabilization of calcium phosphate complexes. These complexes evolve to the formation of ACP, the very first mineral phase in the MVs. Vesicles filled with ACP could directly infiltrate within the collagen fibrils scaffold and then transform to platelet-like crystals. This direct amorphous-apatite transformation has been proposed in vitro using confined polymer domains (Lotsari et al., 2018) and similar structures (i.e. mineralized globules) has been observed in the collagen scaffold of zebra fish fin tails (Mahamid et al., 2010) and avian leg tendon (Zou et al., 2020). Then, MVs could breakdown and release their components to the mineralizing front, either by mechanical stress or actions of phospholipases (Wu et al., 2002). Phospholipases are highly active enzymes in the growth plate (Mebarek et al., 2013). This process could release phospholipid-mineral complexes (associated or not with proteins) to direct the infiltration of biomineral precursor phase in the gap region of collagen through a mechanism similar to the proposed for non-collagenous proteins (Nudelman et al., 2010). Alternatively, these phospholipid-mineral complexes could interact with non-collagenous proteins and then be directed to the collagen matrix for mineralization.
