Abstract
Otitis media (OM) is the most common bacterial infection in children. It remains a major health problem and a substantial socioeconomic burden. Streptococcus pneumoniae (S. pneumoniae) is one of the most common bacterial pathogens causing OM. Innate inflammatory response plays a critical role in host defense against bacterial pathogens. However, if excessive, it has a detrimental impact on the middle ear, leading to the middle ear inflammation, a hallmark of OM. Currently, there has been limited success in developing effective therapeutic agents to suppress inflammation without serious side effects. Here, we show that vinpocetine, an anti-stroke drug, suppressed S. pneumoniae-induced inflammatory response in cultured middle ear epithelial cells as well as in the middle ear of mice. Interestingly, vinpocetine inhibited S. pneumoniae-induced inflammation via upregulating a key negative regulator cylindromatosis (CYLD). Moreover, CYLD suppressed S. pneumoniae-induced inflammation via inhibiting the activation of extracellular signal-regulated kinase (ERK). Importantly, the post-infection administration of vinpocetine markedly inhibited middle ear inflammation induced by S. pneumoniae in a well-established mouse OM model. These studies provide insights into the molecular mechanisms underlying the tight regulation of inflammation via inhibition of ERK by CYLD and identified vinpocetine as a potential therapeutic agent for suppressing the inflammatory response in the pathogenesis of OM via upregulating negative regulator CYLD expression.
Keywords: Inflammation, vinpocetine, CYLD, Streptococcus pneumoniae, otitis media
Introduction
Otitis media (OM) is the most common childhood bacterial infection and the leading cause of conductive hearing loss (1–3). Annually there are 24.5 million visits to physician’s offices in the U.S. (4, 5). Over $5 billion is spent annually for the care of OM (5–7). Since OM causes hearing loss during a crucial period for speech and language development, children with frequent middle ear infections may suffer speech and language disabilities (1–3). Streptococcus pneumoniae (S. pneumoniae) is a major gram-positive bacterium causing OM (2, 8, 9). Current S. pneumoniae vaccines have a limited impact on OM, and inappropriate antibiotic use increased antibiotic-resistance (9–12). Thus, there is an urgent need for the development of new therapeutic strategies for treating OM. Inflammation is a major hallmark of OM (1–3). Innate inflammatory response plays a critical role in host defense against bacteria (13–15). However, if uncontrolled, excessive inflammatory response often causes immunopathology and tissue damage in the middle ear, leading to conductive hearing loss (16–18). Thus, the overactive innate inflammatory response must be tightly regulated. The key regulators and the underlying mechanisms involved in tight control of innate immune response in OM remain largely unknown. Elucidating these mechanisms is thus critical for developing novel therapeutic strategies to control overactive the inflammatory response.
The drug repositioning, also known as drug repurposing, is the application of already existing drugs to new diseases. It has several advantages over the traditional discovery of new drugs including reduced clinical trial risk, time and costs (19–21). Indeed, there have been an increasing number of such successful examples in drug repositioning (19–21). For example, sildenafil was originally approved for angina and has been repositioned for treating erectile dysfunction and pulmonary arterial hypertension (20, 22). Thus, drug repositioning represents an advantageous strategy with reduced safety risk and a higher success rate for clinical use.
Vinpocetine, a derivative alkaloid vincamine extracted from the periwinkle plant, has long been clinically used for treating cerebrovascular disorders including stroke and dementia (23–25). Its side effects at the therapeutic doses in adults and children were reported rarely in the literature (26–30). Thus, vinpocetine has constantly attracted scientists and clinicians to seek its novel therapeutic applications as well as its underlying molecular mechanism of actions (25). Our previous studies have shown that vinpocetine inhibits tumor necrosis factor-α (TNF-α)- and lipopolysaccharide (LPS)-induced inflammatory response via targeting IκB kinase (IKK)/NF-κB-dependent pathway in vitro and in vivo (31). Moreover, vinpocetine inhibits S. pneumoniae-induced mucin MUC5AC up-regulation in the pathogenesis of OM (32). However, it remains unclear if vinpocetine also suppresses S. pneumoniae-induced inflammatory response in the middle ear.
In this study, we showed that vinpocetine suppressed S. pneumoniae-induced inflammatory response in the middle ear. Interestingly, vinpocetine enhanced S. pneumoniae-induced expression of cylindromatosis (CYLD), a key negative regulator for inflammation. Vinpocetine inhibited S. pneumoniae-induced inflammatory response via inhibition of mitogen-activated protein kinase (MAPK) extracellular signal-regulated kinase (ERK) by CYLD. Importantly, the post-infection administration of vinpocetine markedly suppressed inflammation induced by S. pneumoniae in a well-established mouse OM model. Thus, the present study provides new insights into the molecular mechanisms underlying the tight regulation of inflammatory response in S. pneumoniae-induced OM and identified the new role of vinpocetine in suppressing bacteria-induced OM pathogenesis via upregulating negative regulator CYLD.
Materials and Methods
Reagents and antibodies
Vinpocetine was purchased from Tocris Bioscience. We also tested vinpocetine solution manufactured by pharmaceutical companies for injection, and we found that vinpocetine purchased from both sources exhibited equivalent potency at inhibition of S. pneumoniae-induced inflammation. PD98059 was purchased from Enzo Life Sciences. Antibodies against phospho-ERK1/2 (Thr-202/Tyr-204, #9101), total ERK1/2 (#9102), anti-rabbit HRP-linked antibody (#7074), and anti-mouse HRP-linked antibody (#7076) were purchased from Cell Signaling Technology. Antibodies against CYLD (sc-74435), β-actin (sc-8432), and FITC-conjugated goat anti-mouse IgG (sc-2010) were purchased from Santa Cruz Biotechnology.
Bacterial strains and culture condition
Clinical OM isolates of S. pneumoniae strain 6B, 19F and 23F as well as the well-characterized D39 were used in this study (32–34). All the S. pneumoniae strains were grown on chocolate agar plate and in Todd-Hewitt broth supplemented with 0.5% yeast extract (THY) at 37°C in 5% CO2 overnight. S. pneumoniae were prepared as described previously (32–34) for in vitro and in vivo experiments. For the all in vitro experiments, S. pneumoniae were treated at multiplicity of infection (MOI) of 5. Cells were stimulated with S. pneumoniae for 6 h, or otherwise as indicated. For experiments with inhibitors, cells were pre-treated with vinpocetine or PD98059 for 1 h followed by S. pneumoniae stimulation. The solvent dimethyl sulfoxide (DMSO) was used as control.
Cell culture
All media described below were supplemented with 10% FBS (Sigma-Aldrich) and Pen/Strep (100 units/ml penicillin and 0.1 mg/ml streptomycin; Corning). Human middle ear epithelial cells (HMEEC) were maintained as described previously (35). All cells were cultured at 37°C in 5% CO2.
Real-time quantitative RT-PCR (Q-PCR) analysis
Total RNA was isolated with TRIzol reagent (Invitrogen) by following the manufacturer’s instruction. The reverse transcription reaction was performed using PrimeScript reverse transcription reagent kit (Takara) following the manufacturer’s instruction. For Q-PCR analysis, reactions were amplified and quantified using SYBR Green Universal Master Mix reagent and Applied Biosystems StepOnePlus Real-Time PCR System (Applied Biosystems) as described previously (35, 36). The relative quantities of mRNAs were obtained by using the comparative Ct method and were normalized using human cyclophilin or mouse glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as an endogenous control. The primer sequences used are described in Supplemental Table 1.
Plasmids and transfections
The expression plasmids of Flag-tagged CYLD wild-type (WT) and dominant-negative (DN) mutants of ERK1 and ERK2 have been previously described (32, 34, 37). All transient transfections were carried out in triplicate using TransIT-2020 reagent (Mirus) following the manufacturer’s instruction. Empty vector was transfected as control. Cells were assayed 48 h after transfection.
RNA-mediated interference
Human short interfering RNA (siRNA) oligos for CYLD (siCYLD, L-004609–00) and control siRNA oligos (D-001810–10) were purchased from Dharmacon, and knockdown of CYLD using siCYLD was performed using Lipofectamine RNAiMAX (Invitrogen) following the manufacturer’s instruction. Cells were transfected with 10 nM siRNA and assayed 48 h after transfection.
Western blot analysis
Western blot analysis was performed as described previously and following the manufacturer’s instruction (35, 38). Whole-cell lysate was separated in 8 or 10% SDS-PAGE gel, and transferred to polyvinylidene difluoride (PVDF) membrane, then incubated with antibodies against phospho-ERK1/2, total ERK1/2, CYLD, or β-actin. Respective proteins were visualized by using secondary HRP-conjugated rabbit or mouse IgG antibody (Cell Signaling Technology) and the ECL detection system (Amersham ECL Prime Western Blotting Detection Reagent, GE Healthcare).
Mice and animal experiments
Cyld knock-out (KO) mice have been described previously (35, 37, 39), and age-matched (8–12 weeks old) background C57BL/6 mice were used as WT controls. Anaesthetized mice were inoculated transtympanically or transbullarly with S. pneumoniae at a concentration of 5 × 106 CFU (transtympanically) or 2 × 106 CFU (transbullarly) per mouse, and saline was inoculated as control (32, 33). For transbullar inoculation, mice were anesthetized with intraperitoneal (i.p.) injection of ketamine (100 mg/kg) and xylazine (15 mg/kg). Through the incision of the ventral aspect of the neck, the ventral aspect of the bulla was exposed and a hole was drilled with a sterile 29-gauge needle under the surgical microscope. Approximately 10 μL of S. pneumoniae (total 2 × 106 CFU) was injected slowly into the middle ear cavity with a thinner needle. The hole was then sealed with a fat graft harvested from the adjacent neck tissue and the incision stapled. The inoculated mice were then sacrificed at 9 h or 3 days post-inoculation. Eardrums of mice were inspected for signs of middle ear inflammation and photographed for recording pathological changes of eardrum under the video otoscope (MedRx Deluxe, MedRx Inc.). Dissected mouse middle ears were then subjected to total RNA extraction and histological analysis. For inhibition study, mice were pretreated with vinpocetine or PD98059 intraperitoneally 2 h before S. pneumoniae inoculation. For the post-S. pneumoniae inoculation treatment experiments, mice were post-treated with vinpocetine intraperitoneally 2 h after S. pneumoniae inoculation. The treatment was repeated once a day during the experiment. All animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) at Georgia State University.
Histology and immunofluorescence (IF) assay
For histological analysis, formalin-fixed paraffin-embedded mouse middle ear tissues were sectioned (4–5 μm), and then stained with hematoxylin and eosin (H&E) to visualize inflammatory responses and pathological changes in the middle ear as described previously (32, 33, 35). For IF assay, IF detection of CYLD protein was performed using mouse anti-CYLD (Santa Cruz Biotechnology), and FITC-conjugated goat anti-mouse IgG (Santa Cruz Biotechnology) in the paraffin section of mouse middle ear tissues as described previously (35). Images of stained tissue sections were recorded under light- and fluorescence-microscopy systems (AxioVert 40 CFL, AxioCam MRC, and AxioVision LE Image system, Carl Zeiss), and analyzed by using a quantitative image analysis system with the VISIOPHARM Integrator System software version 3.0.8.0 (Visiopharm, Horsholm, Denmark).
Statistical analysis
All experiments were repeated in at least three independent experiments. Data are shown as mean ± SD or mean ± SEM of n determinations. Statistical analysis was assessed by two-tailed unpaired student’s t-test. p < 0.05 was considered statistically significant.
Results
Vinpocetine suppresses S. pneumoniae-induced inflammatory response in vitro and in vivo
To determine whether vinpocetine suppresses S. pneumoniae-induced inflammatory response, we first assessed the effect of vinpocetine on up-regulation of key pro-inflammatory mediators by S. pneumoniae at the mRNA level using a well-established human middle ear epithelial HMEEC cell culture system in vitro by performing real-time quantitative PCR (Q-PCR) analysis. As shown in Fig. 1A, vinpocetine inhibited up-regulation of pro-inflammatory mediators including interleukin (IL)-1β, IL-6, IL-8, and TNF-α mRNA expression induced by S. pneumoniae. Moreover, the inhibitory effect of vinpocetine on up-regulation of pro-inflammatory mediators was also observed in HMEEC cells treated with a variety of S. pneumoniae strains, including common OM pathogens 6B, 19F, and 23F strains as well as a well-characterized strain, D39 (Fig. 1B, Supplemental Fig. 1A), thereby suggesting that the inhibitory effect of vinpocetine on pro-inflammatory mediators is generalizable to most OM-causing strains of S. pneumoniae. We next determined if vinpocetine also inhibits S. pneumoniae-induced inflammatory response in the middle ear of mice in vivo. We previously investigated the effects of vinpocetine (administered i.p.) at 2.5, 5, and 10 mg/kg of body weight on LPS-induced lung inflammation (31). Vinpocetine inhibited LPS-induced up-regulation of TNF-α, IL-1β and MIP-2 in a dose-dependent manner. Notably, vinpocetine at 10 mg/kg of body weight exhibited the most effective inhibitory effect on LPS-induced lung inflammation. The marked effectiveness of vinpocetine at 10 mg/kg of body weight was also confirmed in bacteria-induced up-regulation of mucin MUC5AC in the middle ear of mice (32). Thus, we sought to directly focus on evaluating the effect of vinpocetine (10 mg/kg, i.p.) on S. pneumoniae-induced inflammation in the middle ear of mice. We first assessed the effect of vinpocetine on the induction of pro-inflammatory mediators by S. pneumoniae at the mRNA level in the middle ear of mice by inoculating S. pneumoniae into the middle ear of wild-type (WT) strain C57BL/6 via a trans-tympanic membrane route. As shown in Fig. 1C and Supplemental Fig. 1B, vinpocetine inhibited up-regulation of pro-inflammatory mediators at the mRNA level in the middle ear of mice inoculated with S. pneumoniae (D39, 6B, and 19F) as assessed by performing Q-PCR analysis. Consistent with these results, vinpocetine also inhibited S. pneumoniae-induced mucosal thickening and polymorphonuclear neutrophil infiltration in the middle ear mucosa as assessed by histological analysis (Fig. 1D, 1E). These data demonstrate that vinpocetine suppresses S. pneumoniae-induced inflammatory response in vitro and in vivo.
Figure 1. Vinpocetine suppresses S. pneumoniae-induced inflammatory response in vitro and in vivo.
(A-B) Human middle ear epithelial cells HMEEC were pre-treated with vinpocetine (10 μM) for 1 h, followed by stimulation with S. pneumoniae for 6 h. Relative quantity of IL-1β, IL-6, IL-8 and TNF-α mRNA expression was measured by real-time Q-PCR analysis in HMEEC stimulated with S. pneumoniae strains (A) D39 and (B) 6B, 19F or 23F. (C-E) Mice were pre-treated with vinpocetine (10 mg/kg, i.p.) for 2 h and inoculated transtympanically with S. pneumoniae (5 × 106 CFU per mouse) for 9 h. (C) IL-1β, IL-6, MIP-2 (murine homologue of human IL-8) and TNF-α mRNA expression in the middle ear of mice was measured by real-time Q-PCR analysis. (D) H&E staining of the middle ear tissues from mice was performed (Magnification, ×400; Scale bar, 20 μm) for histological analysis, and (E) the thickness of middle ear mucosa was measured from 15 middle ear sections per experimental group. Data in (A)-(C) and (E) are mean ± SD (A-B, n = 3; C, n = 4; E, n = 15). *p<0.05, **p<0.01, ***p<0.001. Statistical analysis was performed using Student’s t-test. Pictures of the H&E-stained middle ear tissues from one representative experiment are shown in (D) (n = 15). Data are representative of three or more independent experiments. CON, control. Sp, S. pneumoniae. Vinp, vinpocetine.
Vinpocetine suppresses S. pneumoniae-induced inflammatory response via upregulating CYLD
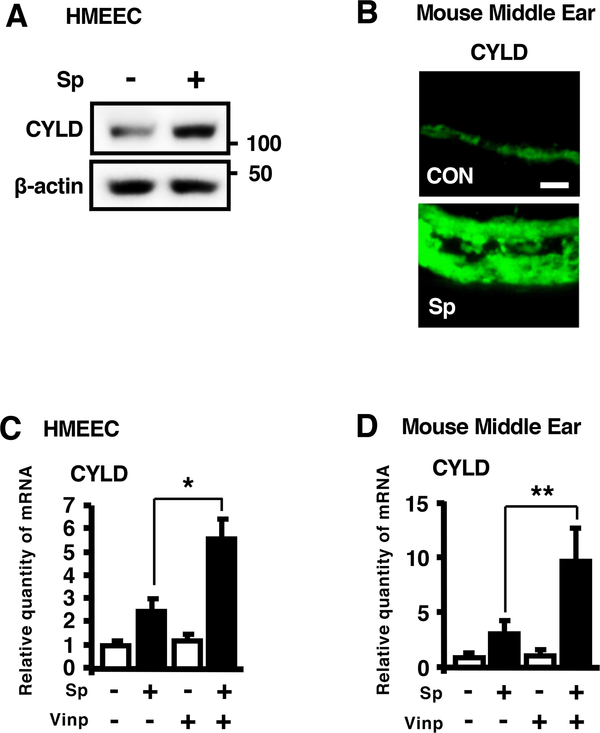
We next sought to determine how vinpocetine suppresses S. pneumoniae-induced inflammatory response. As CYLD has been shown to act as a key negative regulator of bacteria-induced inflammation (35, 40–44), we first evaluated up-regulation of CYLD by S. pneumoniae in vitro and in vivo. Our recent studies have shown that S. pneumoniae induces expression of CYLD at mRNA level in HMEEC in vitro and in the middle ear of mice in vivo (45). Consistent with these studies, CYLD protein expression was also upregulated by S. pneumoniae in HMEEC (Fig. 2A) and in the middle ear of mice (Fig. 2B). We next assessed the effect of vinpocetine on the up-regulation of CYLD induced by S. pneumoniae. Interestingly, vinpocetine significantly enhanced S. pneumoniae-induced up-regulation of CYLD mRNA expression in HMEEC (Fig. 2C) and in the middle ear of mice (Fig. 2D).
Figure 2. Vinpocetine enhances S. pneumoniae-induced upregulation of CYLD expression.
(A) HMEEC were stimulated with S. pneumoniae for 6 h, and cell lysates were analyzed by immunoblotting with the indicated antibodies. (B) Mice were inoculated transtympanically with S. pneumoniae (5 × 106 CFU per mouse) for 9 h, and middle ear tissues were stained with the antibody against CYLD, and probed with FITC-conjugate (Magnification, ×400; Scale bars, 20 μm). (C) HMEEC were pre-treated with vinpocetine (10 μM) for 1 h, followed by S. pneumoniae stimulation for 6 h, and CYLD mRNA expression was measured by real-time Q-PCR analysis. (D) Mice were pre-treated with vinpocetine (10 mg/kg, i.p.) for 2 h and inoculated transtympanically with S. pneumoniae (5 × 106 CFU per mouse) for 9 h. CYLD mRNA expression was measured by real-time Q-PCR analysis in the middle ear of mice. Data in (C) and (D) are mean ± SD (C, n = 3; D, n = 4). *p<0.05. Statistical analysis was performed using Student’s t-test. Pictures of IF-stained middle ear tissues from one representative experiment are shown in (B) (n = 7). Data are representative of three independent experiments. CON, control. Sp, S. pneumoniae. Vinp, vinpocetine.
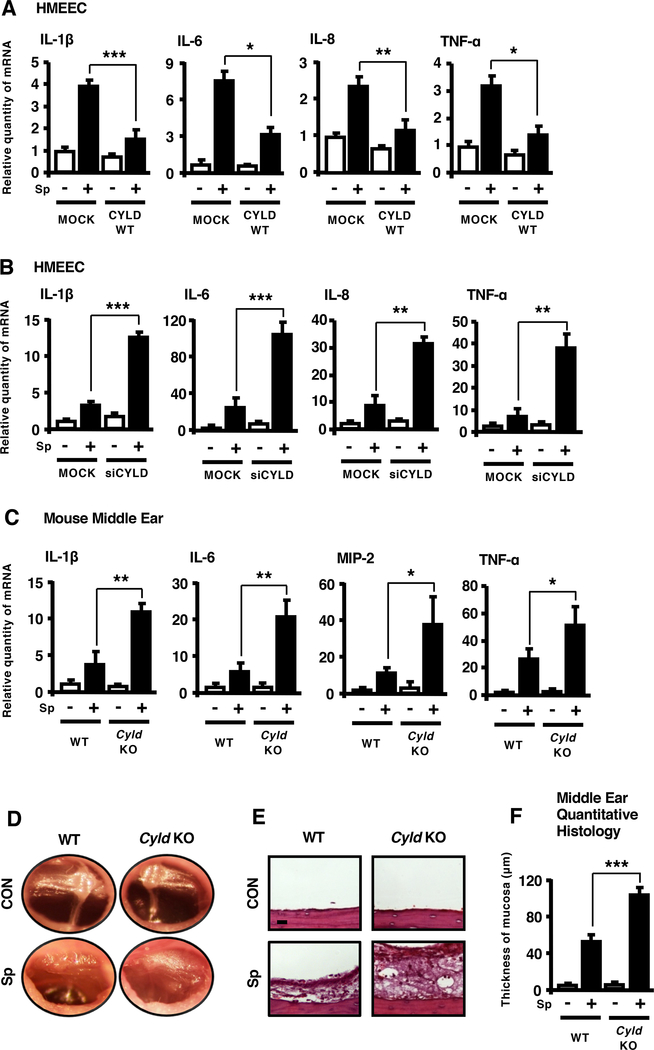
We next determined if CYLD acts as a critical regulator for S. pneumoniae-induced inflammation. As shown in Fig. 3A, expressing CYLD WT inhibited S. pneumoniae-induced IL-1β, IL-6, IL-8 and TNF-α mRNA expression in HMEEC. Conversely, depleting CYLD using short interfering RNA (siRNA) markedly enhanced up-regulation of pro-inflammatory mediators induced by S. pneumoniae (Fig. 3B). Moreover, S. pneumoniae-induced IL-1β, IL-6, IL-8 and TNF-α mRNA expression was enhanced in Cyld knock-out (KO) mice (Fig. 3C), and the typical symptoms of OM including congestion and swelling of tympanic membrane and accumulation of mucous effusion inside the bulla became worse in Cyld KO mice (Fig. 3D). Consistent with these results, S. pneumoniae-induced mucosal thickening and polymorphonuclear neutrophil infiltration in the middle ear mucosa was enhanced in Cyld KO mice (Fig. 3E, 3F). These data suggest that CYLD is a negative regulator for S. pneumoniae-induced inflammatory response in vitro and in vivo.
Figure 3. CYLD negatively regulates S. pneumoniae-induced inflammation in vitro and in vivo.
(A-B) HMEEC transfected with (A) CYLD WT or (B) siCYLD were stimulated with S. pneumoniae for 6 h, and IL-1β, IL-6, IL-8 and TNF-α mRNA expression was measured by real-time Q-PCR analysis. (C-F) WT and Cyld KO mice were inoculated transtympanically with S. pneumoniae (5 × 106 CFU per mouse) for 9 h. (C) IL-1β, IL-6, MIP-2 and TNF-α mRNA expression in the middle ear of mice was measured by real-time Q-PCR analysis. (D) Tympanic cavity of the mouse ear was observed and recorded under the video-otoscope. (E) H&E staining of the middle ear tissues from mice was performed (Magnification, ×400; Scale bar, 20 μm) for histological analysis, and (F) the thickness of middle ear mucosa was measured from 16 middle ear sections per experimental group. Data in (A)-(C) and (F) are mean ± SD (A-C, n = 3; F, n = 16). *p<0.05, **p<0.01, ***p<0.001. Statistical analysis was performed using Student’s t-test. Pictures of the video otoscopy and H&E-stained middle ear tissues from one representative experiment are shown in (D) and (E) (D, n = 6; E, n = 16). Data are representative of three or more independent experiments. Sp, S. pneumoniae. WT, wild-type. KO, knock-out. siCYLD, CYLD siRNA.
Having demonstrated that vinpocetine suppressed S. pneumoniae-induced inflammation and enhanced CYLD up-regulation, it is still unclear whether vinpocetine inhibits S. pneumoniae-induced inflammatory response via upregulating CYLD expression. We first determined if vinpocetine suppresses S. pneumoniae-induced inflammation in CYLD-depleted cells. Interestingly, vinpocetine no longer suppressed S. pneumoniae-induced up-regulation of pro-inflammatory mediators in HMEEC in which CYLD was depleted with CYLD siRNA (Fig. 4A). Moreover, similar results were also confirmed in the mouse model of S. pneumoniae-induced inflammation in the middle ear. As shown in Fig. 4B, vinpocetine no longer inhibited S. pneumoniae-induced up-regulation of pro-inflammatory mediators in the middle ear of Cyld KO mice. As assessed by performing histopathological analysis, vinpocetine also failed to suppress S. pneumoniae-induced inflammatory response including mucosal thickening and polymorphonuclear neutrophil infiltration in Cyld KO mice as compared with WT mice (Fig. 4C, 4D). These data demonstrate that vinpocetine suppresses S. pneumoniae-induced inflammatory response via upregulating the key negative regulator CYLD.
Figure 4. Vinpocetine suppresses S. pneumoniae-induced inflammatory response via upregulating CYLD.
(A) HMEEC transfected with siCON or siCYLD were pre-treated with vinpocetine (10 μM) for 1 h, followed by S. pneumoniae stimulation for 6 h, and IL-8 and TNF-α mRNA expression was measured by real-time Q-PCR analysis. (B-D) WT and Cyld KO mice were inoculated transbullarly with S. pneumoniae (2 × 106 CFU per mouse), and post-treated intraperitoneally with vinpocetine (10 mg/kg) 2 h after S. pneumoniae inoculation. The treatment was repeated at a dose of 10 mg/kg/day during the experiment. The inoculated mice were then sacrificed 3 days after S. pneumoniae inoculation. (B) IL-1β, IL-6, MIP-2 and TNF-α mRNA expression in the middle ear of mice was measured by real-time Q-PCR analysis. (C) H&E staining of the middle ear tissues from mice was performed (Magnification, ×400; Scale bar, 20 μm) for histological analysis, and (D) the thickness of middle ear mucosa was measured from 40 middle ear sections per experimental group. Data in (A) are mean ± SD (n = 3). Data in (B) and (D) are mean ± SEM (B, n = 4; D, n = 40). *p<0.05, **p<0.01, ***p<0.001. n.s., not significant. Statistical analysis was performed using Student’s t-test. Pictures of the H&E-stained middle ear tissues from one representative experiment are shown in (C) (n = 16). Data are representative of three independent experiments. CON, control. Sp, S. pneumoniae. Vinp, vinpocetine. siCYLD, CYLD siRNA. WT, wild-type. KO, knock-out.
ERK1 is required for S. pneumoniae-induced inflammatory response
We next sought to determine how vinpocetine suppresses S. pneumoniae-induced inflammatory response via upregulating CYLD. The MAPK ERK pathway plays an important role in regulating inflammatory responses (46, 47). We previously found that vinpocetine suppresses S. pneumoniae-induced MUC5AC up-regulation via inhibiting ERK (32). Thus, we initially determined the involvement of ERK in S. pneumoniae-induced inflammation in the middle ear. As shown in Fig. 5A, the specific ERK signaling inhibitor PD98059 suppressed S. pneumoniae-induced pro-inflammatory mediators in HMEEC in vitro. As ERK1 and 2 represent the two major isoforms of ERK expressed in the middle ear epithelial cells, we next determined which isoform is involved. Interestingly, expressing ERK1 dominant-negative mutant form (ERK1 DN), but not ERK2 DN form, significantly attenuated S. pneumoniae-induced up-regulation of pro-inflammatory mediators in HMEEC (Fig. 5B). Consistent with these in vitro findings, the administration of PD98059 inhibited S. pneumoniae-induced up-regulation of pro-inflammatory mediators and inflammation including mucosal thickening and polymorphonuclear neutrophil infiltration in the middle ear mucosa (Fig. 5C, 5D). Taken together, our data suggest that ERK1 is required for S. pneumoniae-induced inflammatory response.
Figure 5. ERK1 is required for S. pneumoniae-induced inflammatory response.
(A) HMEEC were pre-treated with PD98059 (10 μM) for 1 h, followed by stimulation of S. pneumoniae for 6 h. Relative quantity of IL-1β, IL-6 and IL-8 mRNA expression was measured by real-time Q-PCR analysis. (B) HMEEC transfected with ERK1 DN or ERK2 DN were stimulated with S. pneumoniae for 6 h, and IL-6, IL-8 and TNF-α mRNA expression was measured by real-time Q-PCR analysis. (C-D) Mice were pre-treated with PD98059 (5 mg/kg, i.p.) for 2 h and inoculated transbullarly with S. pneumoniae (2 × 106 CFU per mouse) for 9 h. (C) IL-1β and TNF-α mRNA expression in the middle ear of mice was measured by real-time Q-PCR analysis. (D) H&E staining of the middle ear tissues from mice was performed (Magnification, ×400; Scale bar, 20 μm) for histological analysis. Data in (A)-(B) are mean ± SD (n = 3). Data in (C) are mean ± SEM (n = 3). *p<0.05, **p<0.01, ***p<0.001. n.s., not significant. Statistical analysis was performed using Student’s t-test. Pictures of the H&E-stained middle ear tissues from one representative experiment are shown in (D) (n = 12). Data are representative of three or more independent experiments. CON, control. Sp, S. pneumoniae. PD, PD98059. DN, dominant negative.
CYLD suppresses S. pneumoniae-induced inflammation by inhibiting ERK
Having established that ERK1 mediates S. pneumoniae-induced inflammation, we sought to determine whether CYLD blocks S. pneumoniae-induced ERK activation. As shown in Fig. 6A, expressing CYLD WT inhibited S. pneumoniae-induced activation of ERK in HMEEC. Conversely, CYLD knockdown significantly enhanced ERK activation induced by S. pneumoniae (Fig. 6B). We next determined whether CYLD suppresses S. pneumoniae-induced ERK-dependent inflammation. Interestingly, the enhancement of S. pneumoniae-induced up-regulation of pro-inflammatory mediators by CYLD knockdown was inhibited by PD98059 in HMEEC (Fig. 6C). Consistent with these in vitro results, enhanced S. pneumoniae-induced up-regulation of pro-inflammatory mediators and inflammation (mucosal thickening and polymorphonuclear neutrophil infiltration) in the middle ear were attenuated by PD98059 administration in Cyld KO mice (Fig. 6D–6F). Taken together, it is evident that CYLD suppresses S. pneumoniae-induced inflammatory response by inhibiting ERK in vitro and in vivo.
Figure 6. CYLD suppresses S. pneumoniae-induced inflammation by inhibiting ERK.
(A-B) HMEEC transfected with (A) CYLD WT or (B) siCYLD were stimulated with S. pneumoniae for 5 min, and cell lysates were analyzed by immunoblotting with the indicated antibodies. (C) HMEEC transfected with siCON or siCYLD were pre-treated with PD98059 (10 μM) for 1 h, followed by S. pneumoniae stimulation for 6 h, and IL-1β, IL-6 and IL-8 mRNA expression was measured by real-time Q-PCR analysis. (D-F) WT and Cyld KO mice were pre-treated with PD98059 (5 mg/kg, i.p.) for 2 h and inoculated transbullarly with S. pneumoniae (2 × 106 CFU per mouse). The treatment of PD98059 was repeated at a dose of 5 mg/kg/day during the experiment. The inoculated mice were then sacrificed 3 days after S. pneumoniae inoculation. (D) IL-6, MIP-2 and TNF-α mRNA expression in the middle ear of mice was measured by real-time Q-PCR analysis. (E) H&E staining of the middle ear tissues from mice was performed (Magnification, ×400; Scale bar, 20 μm) for histological analysis, and (F) the thickness of middle ear mucosa was measured from 20 middle ear sections per experimental group. Data in (C) are mean ± SD (n = 3). Data in (D) and (F) are mean ± SEM (D, n = 4; F, n = 20). *p<0.05, **p<0.01, ***p<0.001. Statistical analysis was performed using Student’s t-test. Pictures of the H&E-stained middle ear tissues from one representative experiment are shown in (E) (n = 16). Data are representative of three independent experiments. CON, control. Sp, S. pneumoniae. WT, wild-type. siCYLD, CYLD siRNA. KO, knock-out. PD, PD98059.
Discussion
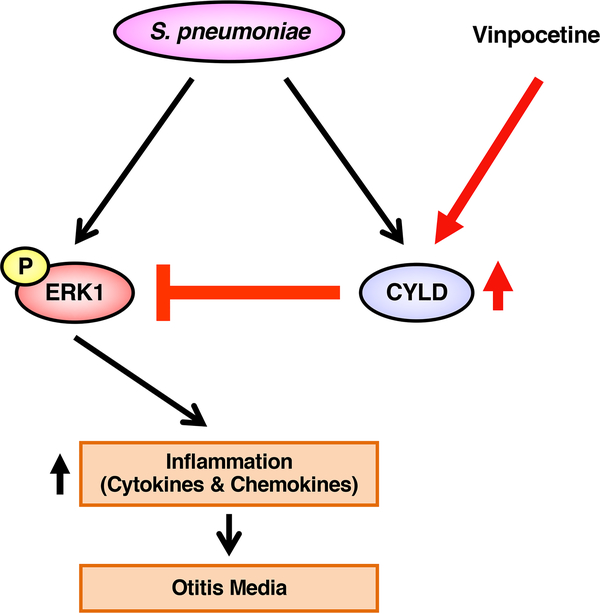
In the present study, we showed that vinpocetine suppressed S. pneumoniae-induced inflammatory response in the middle ear in vitro and in vivo. Interestingly, vinpocetine enhanced up-regulation of key negative regulator CYLD induced by S. pneumoniae. Moreover, vinpocetine no longer suppressed S. pneumoniae-induced inflammation in CYLD-depleted cells and Cyld KO mice. Importantly, vinpocetine inhibited S. pneumoniae-induced inflammatory response via inhibition of MAPK ERK by up-regulating CYLD (Fig. 7). Thus, our study provides new insights into the molecular mechanisms underlying the tight regulation of inflammatory response in S. pneumoniae-induced OM pathogenesis and may help repurpose vinpocetine as a new therapeutic agent for OM.
Figure 7.
Schematic model illustrating that Vinpocetine suppresses S. pneumoniae-induced inflammation via inhibition of ERK1 by CYLD.
The major clinical hallmark of OM is overactive inflammation, which contributes significantly to the middle ear pathology and conductive hearing loss (1–3). Enormous efforts have been put into developing anti-inflammatory agents over the past decades. Although these agents currently available in the clinic exhibited certain efficacy, they often resulted in significant side effects (e.g., the impairment of host defense response), which limited their further clinical use in particular for long-term treatment (48–52). Thus, there is an urgent need for developing novel therapeutic strategies to control overactive inflammatory response without serious side effects. Among various strategies, drug repositioning (drug repurposing) has gained enormous attention from basic research scientists and clinicians due to a number of significant advantages over traditional drug discovery from scratch: [1] the risk of clinical trial failure is lower; [2] time consumption for drug development is shorten; and [3] costs are dramatically reduced (19–21). Taking advantage of the drug repositioning strategy, we previously found that vinpocetine inhibits TNF-α- and LPS-induced inflammatory response in the lung (31). In addition, vinpocetine inhibits S. pneumoniae-induced mucin MUC5AC up-regulation in the middle ear (32). In the present study, we provided the direct evidence that vinpocetine inhibits S. pneumoniae-induced inflammatory response in the middle ear in vitro & in vivo. This finding is of particular translational and therapeutic significance. First, vinpocetine has long been widely used in many countries for the treatment of cerebrovascular diseases (23–25), and a number of clinical studies have demonstrated that vinpocetine at the therapeutic doses in children is safe for long-term use (28–30). Its excellent safety profile makes vinpocetine even more appealing for the treatment of OM as OM is very common in children. Second, our studies have shown that vinpocetine suppresses both overactive inflammation and mucus overproduction, two major hallmarks of OM in the middle ear, thereby achieving a desired therapeutic effect–killing two birds with one stone (32). Moreover, post-infection administration with vinpocetine not only inhibited S. pneumoniae-induced inflammation (Fig. 4B–4D) and mucin up-regulation but also improved hearing and bacterial clearance (32). Collectively, all these advantages make vinpocetine a highly desirable therapeutic agent for treating OM. Moreover, its excellent safety profiles for long-term use also make vinpocetine appealing for the treatment of chronic inflammatory diseases.
Another major finding in the current study is that vinpocetine suppressed S. pneumoniae-induced inflammatory response via up-regulating CYLD, a key negative regulator for inflammation. Previous studies indicate that CYLD plays a critical role in the pathogenesis of numerous diseases including bacteria-induced inflammatory diseases, fibrosis, and cancer (35, 37, 39–44, 53–61). Dysregulated CYLD expression and mutations have been reported under various pathological conditions (56, 57). For example, the expression of CYLD is lower in tumor or damaged tissue upon overactive inflammation than in normal tissue (58–61). It is evident that maintaining appropriate functional activity and expression of CYLD is critical for tightly regulating overactive inflammation and cell proliferation. Thus, it is logical to postulate that up-regulation of CYLD by a pharmacological agent may represent a novel and advantageous therapeutic strategy to restore the lowly expressed CYLD to the levels that allow efficient suppression of overactive pathological conditions (35). Taken together, identifying the new role of vinpocetine in up-regulating CYLD expression may lead to promising therapeutic strategies for the treatment of not only inflammatory diseases such as OM and chronic obstructive pulmonary diseases but also fibrosis and tumors. Future studies will focus on further exploring the therapeutic effects of vinpocetine in various diseases in which CYLD is dysregulated.
Of additional biological significance in our finding is that CYLD inhibits S. pneumoniae-induced MAPK ERK activation and ERK-dependent inflammation. CYLD is a known deubiquitinase (DUB) and has been shown to act as a negative regulator for various signaling pathway by removing lysine 63 (K63)-linked polyubiquitination chains from several key substrates including TNF receptor-associated factors (TRAFs), protein kinase B (Akt), and myeloid differentiation primary response 88 (MyD88) (37, 44, 62, 63). Thus, it is possible that ERK or its upstream molecules including MEK1/2, Raf and MEKK1 (64, 65) may be polyubiquitinated via K63, and CYLD may inhibit the activation of ERK by deubiquitinating ERK or its upstream molecules. We will further pursue studies to determine if the DUB activity of CYLD is required for CYLD-mediated suppression of inflammatory response induced by S. pneumoniae. Because the inhibitory effect of CYLD on S. pneumoniae-induced inflammation was likely both ERK-dependent and -independent (Fig. 6C–6F), we will also further determine how CYLD inhibits S. pneumoniae-induced inflammatory response via directly interacting with and deubiquitinating ERK, or its upstream molecules (MEK1/2, Raf and MEKK1) or other key molecules such as Akt. In addition, it is also possible that CYLD may inhibit ERK activation and inflammation via up-regulation of MAPK phosphatase-1 (MKP-1). MKP-1 has been shown to act as a key negative regulator for inflammation via dephosphorylation and inactivation of ERK (46, 47). We previously demonstrated that vinpocetine suppressed MUC5AC production induced by S. pneumoniae via MKP-1-dependent inhibition of ERK (32). Moreover, CYLD also negatively regulated IL-8 induction by nontypeable Haemophilus influenzae (NTHi), another major OM bacterial pathogen, via MKP-1-mediated ERK inactivation (66). Our future study will focus on elucidating the precise molecular mechanisms underlying how CYLD tightly controls host immune responses via modulating both positive regulator ERK and negative regulator MKP-1.
Supplementary Material
Key Points.
Vinpocetine suppresses S. pneumoniae-induced inflammation in vitro and in vivo.
Vinpocetine inhibits S. pneumoniae-induced inflammation via upregulating CYLD.
CYLD suppresses S. pneumoniae-induced inflammation via inhibiting ERK.
Acknowledgments
This work was supported by grants from National Institutes of Health DC013833 and DC015557 (to J.D.L.), and HL088400 and HL134910 (to C.Y.). J.D.L. is a Georgia Research Alliance Eminent Scholar in Inflammation and Immunity.
Abbreviations
- CYLD
cylindromatosis
- DN
dominant-negative
- HMEEC
Human middle ear epithelial cells
- IF
immunofluorescence
- KO
knock-out
- MKP-1
MAPK phosphatase-1
- OM
Otitis media
- Q-PCR
quantitative PCR
- siRNA
short interfering RNA
- S. pneumoniae
Streptococcus pneumoniae
- WT
wild-type
Footnotes
Disclosures
The authors declare that they have no financial conflicts of interest.
References
- 1.Qureishi A, Lee Y, Belfield K, Birchall JP, and Daniel M. 2014. Update on otitis media - prevention and treatment. Infect Drug Resist 7: 15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schilder AG, Chonmaitree T, Cripps AW, Rosenfeld RM, Casselbrant ML, Haggard MP, and Venekamp RP. 2016. Otitis media. Nat Rev Dis Primers 2: 16063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kurabi A, Pak K, Ryan AF, and Wasserman SI. 2016. Innate Immunity: Orchestrating Inflammation and Resolution of Otitis Media. Curr Allergy Asthma Rep 16: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chartrand SA, and Pong A. 1998. Acute otitis media in the 1990s: the impact of antibiotic resistance. Pediatr Ann 27: 86–95. [DOI] [PubMed] [Google Scholar]
- 5.Gates GA 1996. Cost-effectiveness considerations in otitis media treatment. Otolaryngol Head Neck Surg 114: 525–530. [DOI] [PubMed] [Google Scholar]
- 6.Klein JO 2000. The burden of otitis media. Vaccine 19Suppl 1: S2–8. [DOI] [PubMed] [Google Scholar]
- 7.Allen EK, Manichaikul A, and Sale MM. 2014. Genetic contributors to otitis media: agnostic discovery approaches. Curr Allergy Asthma Rep 14: 411. [DOI] [PubMed] [Google Scholar]
- 8.Murphy TF, Chonmaitree T, Barenkamp S, Kyd J, Nokso-Koivisto J, Patel JA, Heikkinen T, Yamanaka N, Ogra P, Swords WE, Sih T, and Pettigrew MM. 2013. Panel 5: Microbiology and immunology panel. Otolaryngol Head Neck Surg 148: E64–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bergenfelz C, and Hakansson AP. 2017. Streptococcus pneumoniae Otitis Media Pathogenesis and How It Informs Our Understanding of Vaccine Strategies. Curr Otorhinolaryngol Rep 5: 115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pettigrew MM, Alderson MR, Bakaletz LO, Barenkamp SJ, Hakansson AP, Mason KM, Nokso-Koivisto J, Patel J, Pelton SI, and Murphy TF. 2017. Panel 6: Vaccines. Otolaryngol Head Neck Surg 156: S76–S87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zielnik-Jurkiewicz B, and Bielicka A. 2015. Antibiotic resistance of Streptococcus pneumoniae in children with acute otitis media treatment failure. Int J Pediatr Otorhinolaryngol 79: 2129–2133. [DOI] [PubMed] [Google Scholar]
- 12.Korona-Glowniak I, Zychowski P, Siwiec R, Mazur E, Niedzielska G, and Malm A. 2018. Resistant Streptococcus pneumoniae strains in children with acute otitis media- high risk of persistent colonization after treatment. BMC Infect Dis 18: 478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Medzhitov R 2008. Origin and physiological roles of inflammation. Nature 454: 428–435. [DOI] [PubMed] [Google Scholar]
- 14.Nathan C, and Ding A. 2010. Nonresolving inflammation. Cell 140: 871–882. [DOI] [PubMed] [Google Scholar]
- 15.Liew FY, Xu D, Brint EK, and O’Neill LA. 2005. Negative regulation of toll-like receptor-mediated immune responses. Nat Rev Immunol 5: 446–458. [DOI] [PubMed] [Google Scholar]
- 16.Leichtle A, Lai Y, Wollenberg B, Wasserman SI, and Ryan AF. 2011. Innate signaling in otitis media: pathogenesis and recovery. Curr Allergy Asthma Rep 11: 78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li JD, Hermansson A, Ryan AF, Bakaletz LO, Brown SD, Cheeseman MT, Juhn SK, Jung TT, Lim DJ, Lim JH, Lin J, Moon SK, and Post JC. 2013. Panel 4: Recent advances in otitis media in molecular biology, biochemistry, genetics, and animal models. Otolaryngol Head Neck Surg 148: E52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mittal R, Robalino G, Gerring R, Chan B, Yan D, Grati M, and Liu XZ. 2014. Immunity genes and susceptibility to otitis media: a comprehensive review. J Genet Genomics 41: 567–581. [DOI] [PubMed] [Google Scholar]
- 19.Chong CR, and Sullivan DJ Jr. 2007. New uses for old drugs. Nature 448: 645–646. [DOI] [PubMed] [Google Scholar]
- 20.Pushpakom S, Iorio F, Eyers PA, Escott KJ, Hopper S, Wells A, Doig A, Guilliams T, Latimer J, McNamee C, Norris A, Sanseau P, Cavalla D, and Pirmohamed M. 2019. Drug repurposing: progress, challenges and recommendations. Nat Rev Drug Discov 18: 41–58. [DOI] [PubMed] [Google Scholar]
- 21.Corsello SM, Bittker JA, Liu Z, Gould J, McCarren P, Hirschman JE, Johnston SE, Vrcic A, Wong B, Khan M, Asiedu J, Narayan R, Mader CC, Subramanian A, and Golub TR. 2017. The Drug Repurposing Hub: a next-generation drug library and information resource. Nat Med 23: 405–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galie N, Ghofrani HA, Torbicki A, Barst RJ, Rubin LJ, Badesch D, Fleming T, Parpia T, Burgess G, Branzi A, Grimminger F, Kurzyna M, Simonneau G, and Sildenafil G Use in Pulmonary Arterial Hypertension Study. 2005. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med 353: 2148–2157. [DOI] [PubMed] [Google Scholar]
- 23.Bagoly E, Feher G, and Szapary L. 2007. [The role of vinpocetine in the treatment of cerebrovascular diseases based in human studies]. Orv Hetil 148: 1353–1358. [DOI] [PubMed] [Google Scholar]
- 24.Patyar S, Prakash A, Modi M, and Medhi B. 2011. Role of vinpocetine in cerebrovascular diseases. Pharmacol Rep 63: 618–628. [DOI] [PubMed] [Google Scholar]
- 25.Zhang YS, Li JD, and Yan C. 2018. An update on vinpocetine: New discoveries and clinical implications. Eur J Pharmacol 819: 30–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balestreri R, Fontana L, and Astengo F. 1987. A double-blind placebo controlled evaluation of the safety and efficacy of vinpocetine in the treatment of patients with chronic vascular senile cerebral dysfunction. J Am Geriatr Soc 35: 425–430. [DOI] [PubMed] [Google Scholar]
- 27.Feigin VL, Doronin BM, Popova TF, Gribatcheva EV, and Tchervov DV. 2001. Vinpocetine treatment in acute ischaemic stroke: a pilot single-blind randomized clinical trial. Eur J Neurol 8: 81–85. [DOI] [PubMed] [Google Scholar]
- 28.Dutov AA, Gal’tvanitsa GA, Volkova VA, Sukhanova ON, Lavrishcheva TG, and Petrov AP. 1991. [Cavinton in the prevention of the convulsive syndrome in children after birth injury]. Zh Nevropatol Psikhiatr Im S S Korsakova 91: 21–22. [PubMed] [Google Scholar]
- 29.Akopian AV, Pishel’ Ia V, and Khobta VD. 1988. [Characteristics of cerebral blood flow in children with cerebral palsy during the dynamics of treatment]. Zh Nevropatol Psikhiatr Im S S Korsakova 88: 50–53. [PubMed] [Google Scholar]
- 30.Sukhareva MP, Aref’eva NA, and Farkhutdinova LV. 2000. [Pharmacopuncture in combined treatment of children with neurosensory hypoacusis]. Vestn Otorinolaringol: 24–26. [PubMed] [Google Scholar]
- 31.Jeon KI, Xu X, Aizawa T, Lim JH, Jono H, Kwon DS, Abe J, Berk BC, Li JD, and Yan C. 2010. Vinpocetine inhibits NF-kappaB-dependent inflammation via an IKK-dependent but PDE-independent mechanism. Proc Natl Acad Sci U S A 107: 9795–9800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee JY, Komatsu K, Lee BC, Miyata M, O’Neill Bohn A, Xu H, Yan C, and Li JD. 2015. Vinpocetine inhibits Streptococcus pneumoniae-induced upregulation of mucin MUC5AC expression via induction of MKP-1 phosphatase in the pathogenesis of otitis media. J Immunol 194: 5990–5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee J, Komatsu K, Lee BC, Lim JH, Jono H, Xu H, Kai H, Zhang ZJ, Yan C, and Li JD. 2012. Phosphodiesterase 4B mediates extracellular signal-regulated kinase-dependent up-regulation of mucin MUC5AC protein by Streptococcus pneumoniae by inhibiting cAMP-protein kinase A-dependent MKP-1 phosphatase pathway. J Biol Chem 287: 22799–22811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ha U, Lim JH, Jono H, Koga T, Srivastava A, Malley R, Pages G, Pouyssegur J, and Li JD. 2007. A novel role for IkappaB kinase (IKK) alpha and IKKbeta in ERK-dependent up-regulation of MUC5AC mucin transcription by Streptococcus pneumoniae. J Immunol 178: 1736–1747. [DOI] [PubMed] [Google Scholar]
- 35.Komatsu K, Lee JY, Miyata M, Hyang Lim J, Jono H, Koga T, Xu H, Yan C, Kai H, and Li JD. 2013. Inhibition of PDE4B suppresses inflammation by increasing expression of the deubiquitinase CYLD. Nat Commun 4: 1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Susuki-Miyata S, Miyata M, Lee BC, Xu H, Kai H, Yan C, and Li JD. 2015. Cross-talk between PKA-Cbeta and p65 mediates synergistic induction of PDE4B by roflumilast and NTHi. Proc Natl Acad Sci U S A 112: E1800–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lim JH, Jono H, Komatsu K, Woo CH, Lee J, Miyata M, Matsuno T, Xu X, Huang Y, Zhang W, Park SH, Kim YI, Choi YD, Shen H, Heo KS, Xu H, Bourne P, Koga T, Xu H, Yan C, Wang B, Chen LF, Feng XH, and Li JD. 2012. CYLD negatively regulates transforming growth factor-beta-signalling via deubiquitinating Akt. Nat Commun 3: 771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miyata M, Lee JY, Susuki-Miyata S, Wang WY, Xu H, Kai H, Kobayashi KS, Flavell RA, and Li JD. 2015. Glucocorticoids suppress inflammation via the upregulation of negative regulator IRAK-M. Nat Commun 6: 6062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lim JH, Stirling B, Derry J, Koga T, Jono H, Woo CH, Xu H, Bourne P, Ha UH, Ishinaga H, Xu H, Andalibi A, Feng XH, Zhu H, Huang Y, Zhang W, Weng X, Yan C, Yin Z, Briles DE, Davis RJ, Flavell RA, and Li JD. 2007. Tumor suppressor CYLD regulates acute lung injury in lethal Streptococcus pneumoniae infections. Immunity 27: 349–360. [DOI] [PubMed] [Google Scholar]
- 40.Koga T, Lim JH, Jono H, Ha UH, Xu H, Ishinaga H, Morino S, Xu X, Yan C, Kai H, and Li JD. 2008. Tumor suppressor cylindromatosis acts as a negative regulator for Streptococcus pneumoniae-induced NFAT signaling. J Biol Chem 283: 12546–12554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lim JH, Jono H, Koga T, Woo CH, Ishinaga H, Bourne P, Xu H, Ha UH, Xu H, and Li JD. 2007. Tumor suppressor CYLD acts as a negative regulator for non-typeable Haemophilus influenza-induced inflammation in the middle ear and lung of mice. PLoS One 2: e1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang WY, Lim JH, and Li JD. 2012. Synergistic and feedback signaling mechanisms in the regulation of inflammation in respiratory infections. Cell Mol Immunol 9: 131–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lim JH, Ha UH, Woo CH, Xu H, and Li JD. 2008. CYLD is a crucial negative regulator of innate immune response in Escherichia coli pneumonia. Cell Microbiol 10: 2247–2256. [DOI] [PubMed] [Google Scholar]
- 44.Lee BC, Miyata M, Lim JH, and Li JD. 2016. Deubiquitinase CYLD acts as a negative regulator for bacterium NTHi-induced inflammation by suppressing K63-linked ubiquitination of MyD88. Proc Natl Acad Sci U S A 113: E165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lim JH, Ha U, Sakai A, Woo CH, Kweon SM, Xu H, and Li JD. 2008. Streptococcus pneumoniae synergizes with nontypeable Haemophilus influenzae to induce inflammation via upregulating TLR2. BMC Immunol 9: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arthur JS, and Ley SC. 2013. Mitogen-activated protein kinases in innate immunity. Nat Rev Immunol 13: 679–692. [DOI] [PubMed] [Google Scholar]
- 47.Wang X, and Liu Y. 2007. Regulation of innate immune response by MAP kinase phosphatase-1. Cell Signal 19: 1372–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Klein NC, Go CH, and Cunha BA. 2001. Infections associated with steroid use. Infect Dis Clin North Am 15: 423–432, viii. [DOI] [PubMed] [Google Scholar]
- 49.Fardet L, Petersen I, and Nazareth I. 2016. Common Infections in Patients Prescribed Systemic Glucocorticoids in Primary Care: A Population-Based Cohort Study. PLoS Med 13: e1002024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Youssef J, Novosad SA, and Winthrop KL. 2016. Infection Risk and Safety of Corticosteroid Use. Rheum Dis Clin North Am 42: 157–176, ix-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Le Turnier P, Boutoille D, Joyau C, Veyrac G, and Asseray N. 2017. Bacterial infections and NSAIDs exposure? Seek septic complications. Eur J Intern Med 41: e33–e34. [DOI] [PubMed] [Google Scholar]
- 52.Le Bourgeois M, Ferroni A, Leruez-Ville M, Varon E, Thumerelle C, Bremont F, Fayon MJ, Delacourt C, Ligier C, Watier L, Guillemot D, Children A. N. A.-i. D., and G. Childhood Empyema Study. 2016. Nonsteroidal Anti-Inflammatory Drug without Antibiotics for Acute Viral Infection Increases the Empyema Risk in Children: A Matched Case-Control Study. J Pediatr 175: 47–53 e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nikolaou K, Tsagaratou A, Eftychi C, Kollias G, Mosialos G, and Talianidis I. 2012. Inactivation of the deubiquitinase CYLD in hepatocytes causes apoptosis, inflammation, fibrosis, and cancer. Cancer Cell 21: 738–750. [DOI] [PubMed] [Google Scholar]
- 54.Mathis BJ, Lai Y, Qu C, Janicki JS, and Cui T. 2015. CYLD-mediated signaling and diseases. Curr Drug Targets 16: 284–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hellerbrand C, and Massoumi R. 2016. Cylindromatosis--A Protective Molecule against Liver Diseases. Med Res Rev 36: 342–359. [DOI] [PubMed] [Google Scholar]
- 56.Bignell GR, Warren W, Seal S, Takahashi M, Rapley E, Barfoot R, Green H, Brown C, Biggs PJ, Lakhani SR, Jones C, Hansen J, Blair E, Hofmann B, Siebert R, Turner G, Evans DG, Schrander-Stumpel C, Beemer FA, van Den Ouweland A, Halley D, Delpech B, Cleveland MG, Leigh I, Leisti J, and Rasmussen S. 2000. Identification of the familial cylindromatosis tumour-suppressor gene. Nat Genet 25: 160–165. [DOI] [PubMed] [Google Scholar]
- 57.Massoumi R 2011. CYLD: a deubiquitination enzyme with multiple roles in cancer. Future Oncol 7: 285–297. [DOI] [PubMed] [Google Scholar]
- 58.Hayashi M, Jono H, Shinriki S, Nakamura T, Guo J, Sueta A, Tomiguchi M, Fujiwara S, Yamamoto-Ibusuki M, Murakami K, Yamashita S, Yamamoto Y, Li JD, Iwase H, and Ando Y. 2014. Clinical significance of CYLD downregulation in breast cancer. Breast Cancer Res Treat 143: 447–457. [DOI] [PubMed] [Google Scholar]
- 59.Guo J, Shinriki S, Su Y, Nakamura T, Hayashi M, Tsuda Y, Murakami Y, Tasaki M, Hide T, Takezaki T, Kuratsu J, Yamashita S, Ueda M, Li JD, Ando Y, and Jono H. 2014. Hypoxia suppresses cylindromatosis (CYLD) expression to promote inflammation in glioblastoma: possible link to acquired resistance to anti-VEGF therapy. Oncotarget 5: 6353–6364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Espinosa L, Cathelin S, D’Altri T, Trimarchi T, Statnikov A, Guiu J, Rodilla V, Ingles-Esteve J, Nomdedeu J, Bellosillo B, Besses C, Abdel-Wahab O, Kucine N, Sun SC, Song G, Mullighan CC, Levine RL, Rajewsky K, Aifantis I, and Bigas A. 2010. The Notch/Hes1 pathway sustains NF-kappaB activation through CYLD repression in T cell leukemia. Cancer Cell 18: 268–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang LM, Zhou JJ, and Luo CL. 2018. CYLD suppression enhances the pro-inflammatory effects and hyperproliferation of rheumatoid arthritis fibroblast-like synoviocytes by enhancing NF-kappaB activation. Arthritis Res Ther 20: 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sun SC 2010. CYLD: a tumor suppressor deubiquitinase regulating NF-kappaB activation and diverse biological processes. Cell Death Differ 17: 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yoshida H, Jono H, Kai H, and Li JD. 2005. The tumor suppressor cylindromatosis (CYLD) acts as a negative regulator for toll-like receptor 2 signaling via negative cross-talk with TRAF6 AND TRAF7. J Biol Chem 280: 41111–41121. [DOI] [PubMed] [Google Scholar]
- 64.Lim CP, and Cao X. 2001. Regulation of Stat3 activation by MEK kinase 1. J Biol Chem 276: 21004–21011. [DOI] [PubMed] [Google Scholar]
- 65.Johnson GL, Stuhlmiller TJ, Angus SP, Zawistowski JS, and Graves LM. 2014. Molecular pathways: adaptive kinome reprogramming in response to targeted inhibition of the BRAF-MEK-ERK pathway in cancer. Clin Cancer Res 20: 2516–2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang WY, Komatsu K, Huang Y, Wu J, Zhang W, Lee JY, Miyata M, Xu H, and Li JD. 2014. CYLD negatively regulates nontypeable Haemophilus influenzae-induced IL-8 expression via phosphatase MKP-1-dependent inhibition of ERK. PLoS One 9: e112516. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.