Abstract
Background
Leg ulcers are open skin wounds on the lower leg that can last weeks, months or even years. Most leg ulcers are the result of venous diseases. First‐line treatment options often include the use of compression bandages or stockings.
Objectives
To assess the effects of using compression bandages or stockings, compared with no compression, on the healing of venous leg ulcers in any setting and population.
Search methods
In June 2020 we searched the Cochrane Wounds Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL), Ovid MEDLINE (including In‐Process & Other Non‐Indexed Citations), Ovid Embase and EBSCO CINAHL Plus. We also searched clinical trials registries for ongoing and unpublished studies, and scanned reference lists of relevant included studies as well as reviews, meta‐analyses and health technology reports to identify additional studies. There were no restrictions by language, date of publication or study setting.
Selection criteria
We included randomised controlled trials that compared any types of compression bandages or stockings with no compression in participants with venous leg ulcers in any setting.
Data collection and analysis
At least two review authors independently assessed studies using predetermined inclusion criteria. We carried out data extraction, and risk‐of‐bias assessment using the Cochrane risk‐of‐bias tool. We assessed the certainty of the evidence according to GRADE methodology.
Main results
We included 14 studies (1391 participants) in the review. Most studies were small (median study sample size: 51 participants). Participants were recruited from acute‐care settings, outpatient settings and community settings, and a large proportion (65.9%; 917/1391) of participants had a confirmed history or clinical evidence of chronic venous disease, a confirmed cause of chronic venous insufficiency, or an ankle pressure/brachial pressure ratio of greater than 0.8 or 0.9. The average age of participants ranged from 58.0 to 76.5 years (median: 70.1 years). The average duration of their leg ulcers ranged from 9.0 weeks to 31.6 months (median: 22.0 months), and a large proportion of participants (64.8%; 901/1391) had ulcers with an area between 5 and 20 cm2. Studies had a median follow‐up of 12 weeks. Compression bandages or stockings applied included short‐stretch bandage, four‐layer compression bandage, and Unna's boot (a type of inelastic gauze bandage impregnated with zinc oxide), and comparator groups used included 'usual care', pharmacological treatment, a variety of dressings, and a variety of treatments where some participants received compression (but it was not the norm). Of the 14 included studies, 10 (71.4%) presented findings which we consider to be at high overall risk of bias.
Primary outcomes
There is moderate‐certainty evidence (downgraded once for risk of bias) (1) that there is probably a shorter time to complete healing of venous leg ulcers in people wearing compression bandages or stockings compared with those not wearing compression (pooled hazard ratio for time‐to‐complete healing 2.17, 95% confidence interval (CI) 1.52 to 3.10; I2 = 59%; 5 studies, 733 participants); and (2) that people treated using compression bandages or stockings are more likely to experience complete ulcer healing within 12 months compared with people with no compression (10 studies, 1215 participants): risk ratio for complete healing 1.77, 95% CI 1.41 to 2.21; I2 = 65% (8 studies with analysable data, 1120 participants); synthesis without meta‐analysis suggests more completely‐healed ulcers in compression bandages or stockings than in no compression (2 studies without analysable data, 95 participants).
It is uncertain whether there is any difference in rates of adverse events between using compression bandages or stockings and no compression (very low‐certainty evidence; 3 studies, 585 participants).
Secondary outcomes
Moderate‐certainty evidence suggests that people using compression bandages or stockings probably have a lower mean pain score than those not using compression (four studies with 859 participants and another study with 69 ulcers): pooled mean difference −1.39, 95% CI −1.79 to −0.98; I2 = 65% (two studies with 426 participants and another study with 69 ulcers having analysable data); synthesis without meta‐analysis suggests a reduction in leg ulcer pain in compression bandages or stockings, compared with no compression (two studies without analysable data, 433 participants). Compression bandages or stockings versus no compression may improve disease‐specific quality of life, but not all aspects of general health status during the follow‐up of 12 weeks to 12 months (four studies with 859 participants; low‐certainty evidence).
It is uncertain if the use of compression bandages or stockings is more cost‐effective than not using them (three studies with 486 participants; very low‐certainty evidence).
Authors' conclusions
If using compression bandages or stockings, people with venous leg ulcers probably experience complete wound healing more quickly, and more people have wounds completely healed. The use of compression bandages or stockings probably reduces pain and may improve disease‐specific quality of life. There is uncertainty about adverse effects, and cost effectiveness.
Future research should focus on comparing alternative bandages and stockings with the primary endpoint of time to complete wound healing alongside adverse events including pain score, and health‐related quality of life, and should incorporate cost‐effectiveness analysis where possible. Future studies should adhere to international standards of trial conduct and reporting.
Plain language summary
Compression bandages or stockings versus no compression for treating venous leg ulcers
Key messages
Compared with not using compression, compression therapy that uses bandages or stockings to treat venous leg ulcers:
‐ probably heals venous leg ulcers more quickly;
‐ probably increases the number of people whose ulcer has completely healed after 12 months;
‐ probably reduces pain; and
‐ may improve some aspects of people’s quality of life.
However, there is still uncertainty about whether or not compression therapy causes unwanted side effects, and if the health benefits of using compression outweigh its cost.
What are leg ulcers?
Leg ulcers are open skin wounds on the lower leg that can last weeks, months or even years. Most leg ulcers are caused by venous diseases that affect the circulation of blood in leg veins. Venous leg ulcers can cause distress and pain to patients, and can be very costly to the health service.
What did we want to find out?
Standard treatment options for venous leg ulcers often include compression therapy. This involves applying external pressure around the lower leg to help the return of blood from the legs to the heart. Compression therapy uses bandages, stockings or other devices.
We wanted to find out if compression therapy delivered by bandages and stockings compared with no compression:
‐ heals venous leg ulcers;
‐ has any unwanted effects;
‐ improves people’s quality of life;
‐ has health benefits that outweigh the costs (cost‐effectiveness); and
‐ reduces pain.
What did we do?
We searched for randomised controlled trials (clinical studies where the treatment or care people receive is chosen at random). This type of study design provides the most reliable health evidence about the effects of a treatment. We searched for studies that evaluated the effects of any types of compression bandages or stockings compared with no compression in people affected with venous leg ulcers in any care setting. We compared and summarised their results, and rated our confidence in the evidence, based on factors such as study methods and sizes.
What did we find?
We found 14 studies (1391 people, average age: 70.1 years) that lasted on average for 12 weeks. People in eight of the 14 studies were treated in outpatient and community settings. People had venous leg ulcers that had lasted for 22 months on average, and most ulcers had an area between 5 and 20 cm2.
The studies used three types of compression therapy: short‐stretch bandage, four‐layer compression bandage, and Unna's boot (a type of compression bandage containing zinc oxide). These therapies were compared with no compression in forms of 'usual care', pharmacological treatment, a variety of dressings, and a variety of treatments where only some participants received compression (but it was not the norm).
(1) Venous leg‐ulcer healing and unwanted effects
Compared with no compression, the evidence suggests that:
‐ people wearing compression bandages or stockings probably experience complete ulcer healing more quickly; and
‐ more people treated using the compression bandages or stockings are likely to experience complete ulcer healing within 12 months.
However, we did not find clear evidence to tell if using compression bandages or stockings causes any unwanted effects.
(2) Other effects
The evidence suggests that, compared with not using compression, the use of compression bandages or stockings:
‐ probably reduces pain more than not using compression; and
‐ may improve some aspects of people’s quality of life in 12 weeks to 12 months.
However, we are uncertain if the use of compression bandages or stockings results in health benefits that outweigh their costs.
What limited our confidence in the evidence?
Most studies were small (51 people on average) and 10 of the 14 included studies used methods that could introduce errors in their results.
How up‐to‐date is this review?
The evidence in this Cochrane Review is current to June 2020.
Summary of findings
Summary of findings 1. Compression bandages or stockings compared with no compression for treating venous leg ulcers.
| Compression bandages or stockings compared with no compression for treating venous leg ulcers | ||||
|
Patient or population: people with venous leg ulcers Setting: community and acute‐care settings Intervention: compression bandages or stockings Comparison: no compression | ||||
| Outcomes | Impact | № of participants (studies) | Certainty of the evidence (GRADE) | Comments |
| Time‐to‐complete wound healing follow‐up: range 1 day to 12 months | 5 studies (733 participants) with time‐to‐event data: HR 2.17 (95% CI 1.52 to 3.10)a | 733 (5 RCTs) | ⊕⊕⊕⊝ Moderateb | There is probably a shorter time to complete healing of venous leg ulcers in people wearing compression bandages or stockings compared with those not wearing compression |
| Proportion of wounds completely healed during follow‐up follow up: range 1 day to 12 months | 8 studies (1120 participants) with analysable data: RR 1.77 (95% CI 1.41 to 2.21) Two studies (95 participants) without analysable data: 1 study reported 71% of leg ulcers completely healed in short‐stretch bandages and 25% in usual care. 1 study reported 82% of 21 participants with ulcers healed when using compression plus local povidone‐iodine (Betadine) and 62% of 21 participants with ulcers healed when using local povidone‐iodine (Betadine) |
1215 (10 RCTs) |
⊕⊕⊕⊝ Moderatec | People treated with compression bandages or stockings probably have more completely healed venous leg ulcers during follow‐up to 12 months than people not in compression |
| Adverse events follow‐up: range 8 weeks to 12 months | 3 studies (585 participants) with adverse event data that were systematically collected: RR 0.98 (95% CI 0.25 to 3.80) a | 585 (3 RCTs) | ⊕⊝⊝⊝ Very low d,e,f | It is uncertain whether there is any difference in the risk of adverse events associated with using compression and not using compression |
| Participant health‐related quality of life/health status follow‐up: 12 weeks to 12 months | Two studies (426 participants): pooled MD in the total score of the Charing Cross Venous Ulcer Questionnaire (lower scores = better quality of life) −6.87 (95% CI −13.10 to −0.64) between using compression bandages or stockings and no compression, but data analysis showed no difference in the physical component, mental component, and functional status of the SF‐12. Two studies without analysable data (433 participants): 1 study (233 participants) stated that, for most dimensions of the SF‐36 and EuroQol, health status deteriorated over time but was not different between 4‐layer bandages and usual care. 1 study (200 participants) reported a statistical difference in some dimensions of the SF‐36 (including physical function, role‐physical, mental health) and the disease‐specific quality of life instrument for chronic lower limb venous insufficiency (CIVIQ) (physical, social, and global dimensions) but not in others |
859 (4 RCTs) |
⊕⊕⊝⊝ Lowg | Compression bandages or stockings may improve participant health‐related quality of life for some (but not all) aspects during the follow‐up of 12 weeks to 12 months in comparison with no compression |
| Cost effectiveness follow‐up: 12 weeks and 12 months | Two studies without incremental mean cost per incremental gain in benefit: 1 study (53 participants) reported that the short‐stretch bandage was more cost‐effective than usual care as it could be washed and reused repeatedly. 1 study (200 participants) showed that the median cost per leg healed was significantly less for 4‐layer bandages than dressings (P = 0.04). 1 study (233 participants) with incremental mean cost per incremental gain in benefit: incremental cost‐effectiveness ratio = GBP 2.46 (95% CI −31.94 to 99.12) per ulcer‐free week between 4‐layer bandage in leg ulcer clinics and no compression |
486 (3 RCTs) |
⊕⊝⊝⊝ Very low h,i,j | It is uncertain whether compression bandages or stockings are cost effective compared with no compression in wound healing |
| Mean pain score median follow‐up period 12 weeks (minimum 12 weeks maximum 12 months) | Two studies with 426 participants and another study with 69 ulcers reported analysable data, with pain measured by either a 10‐point visual analogue scale or a scale with grades from 1 to 10: pooled MD −1.39 (95% CI −1.79 to −0.98). Two studies without analysable data (433 participants), neither reported the range of scales used: 1 study (233 participants) stated that people treated with 4‐layer bandages were more likely to experience a reduction in leg ulcer pain per month than those using usual care; and another study (200 participants) reported a lower median of pain scores among those using 4‐layer bandages than those using dressings (median 18.8, IQR 6.3 to 37.5; and 31.3, 18.8 to 43.8, respectively; P = 0.14). |
859 participants and 69 ulcers in other participants (5 RCTs) |
⊕⊕⊕⊝ Moderatek | The use of compression probably reduces mean pain score compared with no compression. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; HR: Hazard Ratio; IQR: interquartile range; MD: mean difference; RCT: randomised controlled trial; RR: Risk ratio | ||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||
aOne included study (84 participants with 87 ulcers) in these analyses reported clustered data whilst the other studies reported data by participant; the absolute effect could not be estimated directly. bDowngraded once for risk of bias (one study with clustered data and another small study were at high overall risk of bias in domains other than performance bias, and the other three studies with most of the data in this synthesis were at unclear overall risk of bias). cDowngraded once for risk of bias (six studies having 569/1215 (46.8%) participants were at high risk of bias in the domains other than performance bias and the other four were at unclear risk of bias in some but not all domains). dDowngraded once for risk of bias (two studies with the larger numbers of participants were at high risk of bias in some domains and one study contributing 30.6% weight was at unclear risk of bias). eDowngraded twice for substantial inconsistency as the clustered data were inconsistent with the data reported by participant. fDowngraded once for imprecision because the CIs appeared to include the possibility of both benefit and harm as well as no effect. gDowngraded twice for substantial inconsistency due to the variation of the reported results, particularly in terms of health status. hDowngraded once for risk of bias (one small study was at high overall risk of bias in domains other than performance bias and the other two studies were at unclear risk of bias in some domains). iDowngraded once for indirectness (results from two studies did not appear to be expressed as incremental mean cost per incremental gain in benefit). jDowngraded once for inconsistency in terms of cost‐effectiveness results between studies. kDowngraded once for risk of bias as two (of all six included) studies with 345 participants (a small proportion) were at high overall risk of bias.
Background
Description of the condition
Leg ulcers are open skin wounds on the lower leg (typically below the knee and mainly above the ankle) that can last weeks, months or even years. They occur as a consequence of arterial or venous insufficiency, or both. Less frequently, chronic leg ulceration may occur due to some other disease, such as rheumatoid disease or rarer conditions (Bafaraj 2014). Most leg ulcers are the result of venous disease (Jockenhöfer 2014), where blood flow in the veins is impaired by vein damage, obstruction and calf muscle pump failure (Eberhardt 2014). These problems mean that blood no longer returns efficiently from the legs to the heart and the pressure within the veins rises (Ghauri 2010). The precise chain of events that links high venous pressures with skin breakdown and subsequent chronic wounds is not fully understood.
Leg ulcers of mixed aetiology (those that have more than one cause) usually involve a combination of venous and arterial disease. Open skin ulceration that is due solely to limb ischaemia (a lack of oxygen reaching the leg tissues, i.e. arterial disease) is less common.
Current, accurate estimates of the proportion of leg ulcers due to specific aetiologies can be hard to identify because most studies do not differentiate between venous, arterial or mixed aetiologies of leg ulceration, or do so for each limb but not for each person (Moffatt 2004; Srinivasaiah 2007; Vowden 2009a). Two point‐prevalence surveys undertaken in the north of England estimated that venous ulceration has a prevalence of approximately 0.30 cases per 1000 population in the UK (Cullum 2016; Gray 2018), whilst mixed arterial/venous leg ulceration has a prevalence of 0.11 per 1000 (Cullum 2016). A review of studies of the prevalence of complex wounds suggests that there are limited high‐quality data for estimating the burden of venous leg ulceration in lower‐ and middle‐income countries (Cullum 2016).
A differential diagnosis of the underlying aetiology of a specific leg ulcer is made by taking a clinical history, physical examination, laboratory tests and other assessments (SIGN 2010). Typically, the latter includes an assessment of the arterial blood supply to the leg using the ankle‐brachial pressure index (ABPI), measured using a hand‐held Doppler ultrasound scanner.
Leg ulcers are associated with considerable cost to patients and to healthcare providers. Two systematic reviews summarised the literature on health‐related quality of life in people with leg ulcers (Herber 2007; Persoon 2004). Both included qualitative and quantitative evaluations and reported that the presence of leg ulceration was associated with pain, restriction of work and leisure activities, impaired mobility, sleep disturbance, reduced psychological well‐being and social isolation. Recent research suggests that people with complex wounds, including those with venous leg ulcers, commonly see complete wound healing as their most desirable outcome (Cullum 2016). Leg ulceration is typically a long‐term condition, with periods of healing followed by recurrence stretching over years.
The financial cost of treating a person with an open venous leg ulcer in the UK was estimated at approximately GBP 1700 per year at 2012 prices (Ashby 2014). Nursing time comprises a large part of ulcer treatment costs. A study in Bradford, UK (population approximately 500,000) estimated that for the financial year 2006 to 2007, GBP 1.69 million was spent on dressings and compression bandages, and GBP 3.08 million was spent on nursing time (estimates derived from resource‐use data for all wound types, not just venous leg ulcers) (Vowden 2009b). In the USA the estimated healthcare cost for people with venous leg ulcers was USD 14.9 billion (2012 prices, all payers including Medicare, private, self‐insured) (Rice 2014). In four community wound‐care clinics in Queensland, Australia, the mean weekly cost for each patient with a venous leg ulcer was estimated as AUD 294.72 at 2016/2017 prices for those receiving guideline‐based care (i.e. with at least one ABPI and compression therapy) (Barnsbee 2019).
Description of the intervention
The first‐line treatment for venous leg ulcers is compression therapy in the form of bandages, stockings or other devices (Partsch 2015). This application of external pressure around the lower leg assists venous return (blood flow back to the heart) and reduces venous reflux. This review focuses on the effects of compression delivered by bandages and stockings compared with no compression.
Compression bandages
Bandages are categorised as retention, support or compression, depending on their performance in standardised laboratory tests. Compression bandages are further divided according to the amount of force required to extend them and therefore the level of compression that they can apply to a limb. Furthermore, the laboratory performance of a bandage may not reflect its performance in clinical use, as this depends upon operator training and application technique (specifically, whether the bandage is applied as a spiral or figure‐of‐eight, how many layers are applied and the amount of extension used). Compression systems commonly used for venous leg ulcers are listed below (Thomas 1995).
Class 3a: light‐compression bandages; apply 14 mmHg to 17 mmHg pressure at the ankle when applied in a simple spiral, e.g. Elset (Mölnlycke).
Class 3b: moderate‐compression bandages; apply 18 mmHg to 24 mmHg pressure at the ankle when applied as a simple spiral, e.g. Velkomp (Datt Mediproducts Pvt. Limited).
Class 3c: high‐compression bandages; apply 25 mmHg to 35 mmHg pressure at the ankle when applied as a simple spiral, e.g. Setopress (Mölnlycke), and Elodur forte (BSN Medical).
Class 3d: extra‐high‐compression bandages; apply up to 60 mmHg pressure at the ankle when applied as a simple spiral.
Classification of compression systems
In 2008 a new compression bandage classification system was proposed, based on components rather than the number of 'layers' of bandage (Partsch 2008). The Partsch group recommended that the components of compression, such as orthopaedic wool, crepe bandage or cohesive elastic bandage, should be described. Other recommended classification criteria included sub‐bandage pressure (measured in the medial gaiter area with the patient supine) and the elastic property of the overall compression system. The following are examples of multi‐component bandage systems (listed for illustrative purposes only; not intended as practice recommendations).
Short stretch/inelastic systems: orthopaedic padding plus one or two rolls of short stretch bandage (SSB).
Inelastic paste systems: paste bandage plus support bandage, e.g. Setocrepe (Mölnlycke).
Two‐component bandage systems: orthopaedic padding plus elastic bandage, e.g. 3MTM CobanTM 2 Compression System.
Four‐component bandage systems: orthopaedic padding plus support bandage (crepe) plus class 3a bandage, e.g. PROFORETM compression system (Smith & Nephew).
The earliest Cochrane Review of compression for venous leg ulcers (Cullum 2001) defined different compression systems by the number of layers whereas, in line with the recommendations of the consensus group outlined above, subsequent versions refer to components. Nonetheless, where a trial treatment is the original Charing Cross four‐layer bandage, or a close variant of it, we have continued to use the term 'four‐layer bandage' (4LB), as this is an internationally‐recognised bandage system.
It is more difficult to classify different compression systems in relation to sub‐bandage pressures since, in general, this information is not available from clinical trial reports. In order to gain further insights into the optimal way to classify different compression systems, we consulted with experts in wound management and invited them to complete a survey (informing the previous update of this review) (O'Meara 2012). The survey listed different types of compression against various classifications, and respondents were asked to provide the best choice of classification in their opinion. In addition, free‐text comments were invited. We used the information gleaned from this exercise to classify different types of compression therapy for the previous update of this review (O'Meara 2012).
Compression stockings
Compression stockings (or hosiery) can be used to treat open ulcers and to reduce the risk of recurrence post‐healing. Stockings are classified according to the level of compression they apply to the limb. Importantly, the pressure applied by stockings is subject to less operator variability than bandages.
Class 1: light‐support stockings; provide 14 mmHg to 17 mmHg pressure at the ankle. Used to treat varicose veins.
Class 2: medium‐support stockings; provide 18 mmHg to 24 mmHg pressure at the ankle. Used to treat more severe varicosities, and to prevent venous leg ulcers.
Class 3: strong‐support stockings; provide 25 mmHg to 35 mmHg pressure at the ankle. Used to treat severe chronic hypertension and severe varicose veins, and to prevent venous leg ulcers.
Alongside compression, wound dressings are almost always applied to open ulcers. Dressings protect the surface of the ulcer, absorb exudate and can be antimicrobial. A series of reviews has addressed the comparative effectiveness of dressings for venous ulcers (Norman 2018; O'Meara 2013; O'Meara 2015). Other treatments for venous leg ulcers include venous surgery (removal of incompetent superficial veins (Gohel 2018)) and drugs such as pentoxifylline (Jull 2012).
How the intervention might work
Generally, compression therapy is thought to work by applying an external pressure to the leg which assists venous return (blood flow back to the heart) and reduces venous reflux (Woo 2013). Partsch has suggested that compression:
reduces oedema by reducing capillary filtration, moving fluid from compressed tissues to non‐compressed tissues and improving lymphatic drainage (Partsch 2011); and
reduces the pressure in the veins by increasing venous blood flow and reducing venous pooling (Partsch 2011).
The use of compression to treat venous leg ulcers is not without risk. Whilst Mosti 2012 has suggested that compression may increase arterial inflow, if the applied pressure exceeds the local arterial perfusion pressure then arterial inflow will be reduced, which risks ischaemia.
National clinical guidelines in the UK and USA recommend that all people presenting with a leg ulcer be screened for arterial disease using Doppler‐aided measurement of the ABPI (Bolton 2014; SIGN 2010). Clinically significant arterial disease is often defined as an ABPI of below 0.8. People with venous leg ulceration who have an ABPI of between 0.5 and 0.8 may be eligible to receive modified (reduced) compression (Moffatt 2007).
Why it is important to do this review
Venous leg ulcers have a large impact on people’s lives and incur high costs to health services; compression therapy is currently the first‐line treatment. Since the publication of the original Cochrane Review of compression bandages and stockings for venous leg ulcers (Cullum 2001), the number of relevant randomised controlled trials (RCTs) has more than doubled, the range of compression modalities has increased, and the classification of compression modalities has been refined. We update the evidence from the review (O'Meara 2012) in order to offer up‐to‐date evidence for decision‐makers, and have decided to break down the previous version into separate reviews by compression modality. We will then review all compression modalities together in an overview, which will incorporate a network meta‐analysis (Salanti 2012), in order to rank the different treatments on their individual probabilities of being the most effective compression modalities for healing venous leg ulcers. This particular review provides evidence about the comparison of compression bandages or stockings versus no compression.
Objectives
To assess the effects of using compression bandages or stockings, compared with no compression, on the healing of venous leg ulcers in any setting and any population.
Methods
Criteria for considering studies for this review
Types of studies
We included published and unpublished RCTs, including cluster‐RCTs and cross‐over trials, irrespective of language of report. We excluded studies which used quasi‐randomised methods to allocate treatment (e.g. alternation or odd/even case numbers). We included trials if the application of compression was the only systematic difference between study arms.
Types of participants
We included randomised controlled trials which recruited people of any age with venous leg ulceration (which may also be described as 'stasis' or 'varicose' ulceration) in any care setting. As the method of diagnosis of venous ulceration could vary between studies, we applied no standardised definition, but each study had to refer to the use of compression for venous ulcers.
We included studies that recruited participants with a variety of wound types, including venous leg ulcers, if: a) the allocation of participants was stratified by wound type and included 'venous leg ulcer' as a group and results were presented (or available from the study authors) separately for this group; or b) studies included participants with non‐venous leg ulcers, but these made up a maximum of 25% of the total study population and we assumed that any treatment effect applied to people with venous ulcers. We excluded RCTs which only recruited people with non‐venous leg ulcers (e.g. arterial, or mixed) from the review.
Types of interventions
We included trials which compared the use of any compression bandage or stocking or any combination of compression with no compression (e.g. standard care, simple retention bandages, dressings alone) in participants with venous leg ulcers. We excluded trials where intermittent pneumatic compression was the mode of compression being evaluated, as this is the focus of another Cochrane Review (Nelson 2014).
Types of outcome measures
Assessment of outcomes at different follow‐up periods
We grouped outcome data using the following time categories; we used our judgement to decide whether statistical pooling within these categories was appropriate.
Short term: up to eight weeks.
Medium term: between eight and 24 weeks.
Long term: more than 24 weeks.
Where relevant, we reported outcomes at the latest time point available (assumed to be length of follow‐up, if not specified) and the time point specified in the methods as being of primary interest (if this was different from the latest time point available).
Primary outcomes
The primary effectiveness outcome for this review was ulcer healing. Trialists used a range of different methods for measuring and reporting this outcome. RCTs that reported one or more of the following were considered as providing the most relevant and rigorous measures of wound healing.
Time to complete wound healing (correctly analysed using survival, time‐to‐event approaches or median (or mean) time to healing, if it was clear that all wounds were healed at follow‐up).
Proportion of wounds completely healed during follow‐up (frequency of complete healing).
We used the study authors' definitions of complete wound healing, and reported these where possible. Where both the complete wound‐healing outcomes above were reported for a study, we presented both and gave precedence to time‐to‐healing in our interpretation where possible.
The primary safety outcome for the review was all reported adverse events. Where reported, and a clear methodology for the collection of adverse event data had been provided, we extracted data for all serious adverse events and all non‐serious adverse events. We preferred to focus on the numbers of participants with adverse events in each study arm; the methodology should make it clear whether events were reported at the participant level or, if multiple events/people were reported, that an appropriate adjustment was made for data clustering.
Secondary outcomes
Participant health‐related quality of life/health status: measured using a standardised generic questionnaire such as EQ‐5D (Herdman 2011), SF‐36 (Ware 1992), SF‐12 (Ware 1996) or SF‐6 (Craig 2013), or wound‐specific questionnaires such as the Cardiff Wound Impact Schedule (Price 2004). We did not include ad hoc measures of quality of life that were not likely to be validated and would not be common to multiple trials.
Cost effectiveness: within‐trial cost‐effectiveness analysis comparing mean differences in effects with mean cost differences between the two arms. Data extracted could be incremental mean cost per incremental gain in benefit (incremental cost‐effectiveness ratio (ICER)). We also extracted other relative cost‐effectiveness measures (e.g. net monetary benefit) and cost analysis findings.
Mean pain score (including pain at dressing change): measured as a continuous outcome using a validated scale such as a visual analogue scale (VAS) or other recognised measurement instrument.
For changes to this section please see Differences between protocol and review.
Search methods for identification of studies
Electronic searches
We searched the following electronic databases to identify reports of relevant clinical trials:
the Cochrane Wounds Specialised Register (searched 30 June 2020);
the Cochrane Central Register of Controlled Trials (CENTRAL; 2020, Issue 5) in the Cochrane Library (searched 30 June 2020);
Ovid MEDLINE including In‐Process & Other Non‐Indexed Citations (1946 to 30 June 2020);
Ovid Embase (1974 to 30 June 2020);
EBSCO CINAHL Plus (Cumulative Index to Nursing and Allied Health Literature; 1937 to 30 June 2020).
The search strategies for the Cochrane Wounds Specialised Register, CENTRAL, Ovid MEDLINE, Ovid Embase and EBSCO CINAHL Plus can be found in Appendix 1. We combined the Ovid MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision) (Lefebvre 2021). We combined the Embase search with the Ovid Embase filter developed by the UK Cochrane Centre (Lefebvre 2021). We combined the CINAHL Plus search with the trial filter developed by Glanville 2019 (Differences between protocol and review). There were no restrictions by language, date of publication or study setting.
We also searched the following trials registries:
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov) (searched 30 June 2020);
World Health Organization (WHO) International Clinical Trials Registry Platform (apps.who.int/trialsearch/) (searched 28 August 2019). We could not search this database 30 June 2020 as it was unavailable due to heavy traffic generated by the COVID‐19 situation.
Search strategies for clinical trial registries can be found in Appendix 1.
Searching other resources
We identified other potentially eligible trials or ancillary publications by carrying out a search of the reference lists of retrieved included trials, as well as relevant systematic reviews, meta‐analyses and clinical practice guidelines for leg ulcers (Australian Wound Management Association 2011; Bolton 2014; Franks 2016; Marston 2016; O'Donnell 2014; SIGN 2010; Wittens 2015).
When necessary, we contacted authors of key papers and abstracts to request further information about their trials.
We did not perform a separate search for adverse effects of interventions used, but considered adverse effects described in included studies only.
Data collection and analysis
We carried out data collection and analysis according to the methods stated in the published protocol (Shi 2019), which were based on the Cochrane Handbook for Systematic Reviews of Interventions (McKenzie 2021). Changes from the protocol or previous published versions of the review are documented in Differences between protocol and review.
Selection of studies
Two review authors independently assessed the titles and abstracts of the citations retrieved by the searches for relevance. After this initial assessment, we obtained full‐text copies of all studies considered to be potentially relevant. Two review authors independently checked the full papers for eligibility, with disagreements resolved by discussion and, where required, the input of a third review author. Where the eligibility of a study was unclear we contacted study authors. We recorded all reasons for exclusion of studies for which we obtained full‐text copies. We completed a PRISMA flowchart to summarise this process (Liberati 2009).
Where studies were reported in multiple publications or reports, we obtained all publications. Whilst the study was included only once in the review, obtaining all publications maximised the amount of data we extracted. We also examined any relevant retraction statements and errata for information.
Data extraction and management
We extracted and summarised details of the eligible studies using a data extraction sheet. One review author extracted data and another review author independently checked all data (Differences between protocol and review). We resolved any disagreements through discussion, consulting a third review author where required. Where data were missing from reports, we contacted the study authors to obtain this information.
Where possible we extracted the following data:
country of origin;
trial design (e.g. parallel, cluster);
study start date and end date;
study population, including key related medical histories, diagnosis methods, the aetiology of leg ulcers (e.g. post‐thrombotic syndrome, varicose veins, chronic venous reflux), the onset or recurrence of leg ulcers, and the location of leg ulcers;
care setting;
eligibility criteria and key baseline participant data (total number of participants, age, sex, duration of leg ulcers, baseline leg ulcer area);
details of the interventions, including compression devices used, and duration of interventions applied;
descriptions of any co‐interventions or standard care;
follow‐up period;
unit of randomisation (e.g. leg ulcer, limb, or participant);
numbers of participants randomised to each intervention;
unit of analyses;
number of ulcers per person;
primary and secondary outcomes measured;
data about time to complete wound healing: hazard ratio (HR) and its 95% confidence interval (CI), or any data that will allow its calculation (Parmar 1998; Tierney 2007);
data on the proportion of wounds completely healed during follow‐up: odds ratio (OR) and its 95% CI, or numbers of participants who have leg ulcers completely healed in each arm, both at the latest time point and (if different) at another time specified as of primary interest in the Methods section;
whether a Kaplan Meier plot was displayed;
missing data rates per arm, and reasons for 'missingness', including the number of people who died;
publication status of study; and
source of funding for trial.
Assessment of risk of bias in included studies
Two review authors independently assessed each included study using the Cochrane tool for assessing risk of bias (Higgins 2017). This tool addresses seven specific domains: sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete data, selective outcome reporting, and other issues (Differences between protocol and review). In this review we included unit‐of‐analysis issues under the domain of 'other issues', for example where a cluster‐randomised trial had been undertaken but analysed at the individual level in the study report. We assessed blinding of participants and personnel, blinding of outcome assessment, and incomplete outcome data for each of the review outcomes separately. We note that blinding of participants and personnel as to whether or not participants had been allocated to compression is impossible and therefore performance bias is a risk. Performance bias may be introduced when awareness of treatment allocation results in deviations from intended interventions and/or differential co‐interventions use or care between groups not specified in study protocol which may influence outcomes. We scrutinised study reports and protocols (where available) to understand if, and how, studies attempted to minimise and document protocol deviations and differential care/co‐interventions compensated for this: for example, the study protocol might have been used to highlight the need to balance co‐interventions as well as potentially measuring and reporting this.
We assessed risk of bias for each domain as either low risk, high risk or unclear risk. Since wound healing is a subjective outcome, unblinded outcome assessment represents a high risk of bias (Hróbjartsson 2012). We therefore recorded only open intervention studies with blinded outcome assessment as being at low risk of detection bias.
We resolved all disagreements in risk‐of‐bias assessment by discussion and, where required, we sought the input of a third review author. Where possible, useful and feasible, when a lack of reported information resulted in a judgement of unclear risk of bias, we contacted study authors for clarification. We present our assessment of risk of bias using two risk‐of‐bias summary figures; one is a summary of bias for each item across all studies, and the second shows a cross‐tabulation of each trial by all of the risk‐of‐bias items. We classified studies with an assessment of high risk of bias for one or more of the seven domains as being at high risk of bias overall for the specified outcome (Differences between protocol and review).
For trials using cluster randomisation, we planned to consider the risk of bias in relation to: recruitment bias, baseline imbalance, loss of clusters, incorrect analysis and comparability with individually‐randomised trials (Higgins 2017; Eldridge 2016) (Appendix 2). However, we did not include any studies with a cluster design.
Measures of treatment effect
For dichotomous outcomes (e.g. proportion of participants who have wounds completely healed during follow‐up), we present the risk ratio (RR) with 95% confidence intervals (CIs). For continuous outcomes we present the mean difference (MD) with 95% CIs, for trials that used the same assessment scale. If trials that reported continuous data used different assessment scales, we present the standardised mean difference (SMD) with 95% CIs.
Time‐to‐event data (e.g. time to complete wound healing) are reported as hazard ratios (HRs) where possible, in accordance with the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2021). If studies reporting time‐to‐event data (e.g. time to healing) did not report a HR, then, when feasible, we estimated this using other reported outcomes (such as numbers of events) through the application of available statistical methods (Parmar 1998; Tierney 2007). We only considered median time to healing without survival analysis as a valid outcome if reports specified that all leg ulcers had healed (i.e. if the trial authors treated time‐to‐healing as a continuous measure, as there was no censoring).
Unit of analysis issues
We noted whether trials presented outcomes at the level of the leg ulcer, the limb or the participant, and whether there may have been multiple ulcers reported for the same participant. One included study (Kikta 1988) randomised at the participant level and outcomes were measured at the wound level, e.g. leg ulcer healing; we treated the participant as the unit of analysis when the number of leg ulcers assessed appeared to be equal to the number of participants (e.g. one leg ulcer per person).
A particular unit‐of‐analysis issue may occur in trials if randomisation was carried out at the participant level, with the allocated treatment used on multiple leg ulcers per participant, but data are presented and analysed per leg ulcer (clustered data). We noted whether data for multiple ulcers on a participant were (incorrectly) treated as independent within a study, or were analysed using within‐participant analysis methods. If clustered data were incorrectly analysed, we recorded this as part of the risk‐of‐bias assessment. For an individually‐randomised trial, such data on multiple leg ulcers were collected and analysed where applicable:
only in a proportion of participants; in this case, we only extracted and presented relevant data but did not treat the trial as a cluster trial to seek for an analysis because the trial incorrectly included a mixture of individual and clustered data; or
in all participants; in this case, we planned to treat the trial as a cluster trial and incorporate relevant data in meta‐analyses if the trial was analysed correctly. Where a cluster trial was incorrectly analysed, we planned to record this in the risk‐of‐bias assessment. Where possible we planned to approximate the correct analyses based on guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021).
Useful information for approximating the correct analyses include:
the number of clusters randomised to each arm or the average size of each cluster;
the outcome data ignoring the cluster design; and
an estimate of the intracluster correlation coefficient (ICC).
However, we did not include any cluster trials. As noted above, we analysed data that were available in one of three included studies that randomised individual participants but reported data by ulcers (i.e. clustered data).
For cross‐over trials, we planned to only consider outcome data at the first intervention phase (i.e. prior to cross‐over) as eligible. However, we were not able to obtain such data from the authors of the only cross‐over trial included.
Dealing with missing data
It is common for there to be data missing from trial reports. Excluding participants from the analysis post‐randomisation or ignoring participants who are lost to follow‐up compromises the randomisation and potentially introduces bias into the trial. If we thought that study authors might be able to provide some missing data, we attempted to contact them, but it is likely that data will often be missing because of loss to follow‐up.
In individual studies, when data for the proportion of leg ulcers healed were presented, we assumed that randomly‐assigned participants not included in an analysis had an unhealed leg ulcer at the end of the follow‐up period (i.e. they were considered in the denominator but not in the numerator). We examined the impact of this assumption through doing a sensitivity analysis (see Sensitivity analysis) in which we assumed participants with missing data had a healed leg ulcer (i.e. they were included in both the numerator and the denominator). When a trial did not specify participant group numbers before dropout, we presented only complete‐case data. For the time‐to‐healing analysis using survival analysis methods, dropouts should be accounted for as censored data. Hence all participants contribute to the analysis. We acknowledged that such analysis assumes that dropouts were missing at random and that there was no pattern of 'missingness'.
We presented data for all categorical secondary outcomes as a complete‐case analysis. For continuous secondary outcome variables (i.e. quality of life, pain score), we presented available data from the study reports/study authors and did not impute missing data. We planned to calculate measures of variance when these were missing (Deeks 2021) or we planned to contact study authors, where possible. Where these measures of variation remained unavailable, we planned to exclude the study from any relevant meta‐analyses. However, we did not carry out these because all relevant included studies either fully reported the measures of variance or only reported narrative findings.
Assessment of heterogeneity
Assessment of heterogeneity can be a complex, multi‐faceted process. Firstly, we considered clinical and methodological heterogeneity, that is the degree to which the included studies varied in terms of participants' characteristics (e.g. mean age, proportion of participants by sex, methods of diagnosing leg ulcers), interventions (e.g. delivery approaches of compression systems), outcome definitions and other characteristics such as duration of follow‐up. This assessment of clinical and methodological heterogeneity was supplemented by information about statistical heterogeneity. We assessed statistical heterogeneity using the Chi2 test (a significance level of P value less than 0.10 was considered to indicate statistically significant heterogeneity) in conjunction with the I2 measure (Higgins 2003). I2 examines the percentage of total variation across RCTs that is due to heterogeneity rather than chance (Higgins 2003). Very broadly, we considered that I2 values of 25% or less did not indicate important heterogeneity, and values of more than 75% indicated considerable heterogeneity (Deeks 2021; Higgins 2003).
These statistical tests are recognised to be underpowered and should only be used as an indicator of heterogeneity. Clinical, methodological and statistical heterogeneity should therefore be considered together for the overall assessment of heterogeneity. Where there was no clinical or statistical heterogeneity, we used a fixed‐effect model. In the absence of clinical heterogeneity and in the presence of some statistical heterogeneity (I2 over 50%), we used a random‐effects model; however, we did not anticipate pooling data across studies where heterogeneity was considerable (I2 over 75%). Where there was evidence of considerable heterogeneity we explored this further if required: see Data synthesis.
Assessment of reporting biases
Reporting biases arise when the dissemination of research findings is influenced by the nature and direction of results. Publication bias is one of a number of possible causes of small‐study effects, that is, a tendency for estimates of the intervention effect to be more beneficial in smaller RCTs. Funnel plots allow a visual assessment of whether small‐study effects may be present in a meta‐analysis. A funnel plot is a simple scatter plot of the intervention effect estimates from individual RCTs against some measure of each trial’s size or precision (Page 2021). Funnel plots are only informative when there are a substantial number of studies included in an analysis; we planned to present funnel plots for meta‐analyses that included at least 10 RCTs, using Review Manager 5 (RevMan 2020). However, we did not produce any funnel plots because all the meta‐analyses we conducted contained fewer than 10 studies.
Data synthesis
We summarised details of included studies in a narrative review according to the comparison between intervention and comparator, the participants, and the outcome measurement including the follow‐up duration. We considered clinical and methodological heterogeneity and undertook pooling if studies appeared appropriately similar in terms of participants, intervention comparison, and outcome assessment including follow‐up duration. Where studies were not similar enough for pooling, we present the results of included studies narratively.
Once we had decided to pool the results of individual studies, we used a random‐effects approach for meta‐analysis. Conducting meta‐analysis with a fixed‐effect model in the presence of even minor heterogeneity may provide overly‐narrow confidence intervals. We planned to only use a fixed‐effect approach when clinical and methodological heterogeneity was assessed to be minimal, with the assumption that a single underlying treatment effect was being estimated. We used Chi2 and I2 to quantify heterogeneity but not to guide the choice of model for meta‐analysis. We exercised caution when meta‐analysed data were at risk of small‐study effects, because a random‐effects model may be unsuitable. In this case, or where there were other reasons to question the selection of a fixed‐effect or random‐effects model, we assessed the impact of the approach using sensitivity analyses to compare results from alternate models (Thompson 1999). We reported any evidence that suggested that the use of a particular model might not be robust.
We produced pooled estimates of the treatment effect using Review Manager 5 (RevMan 2020) and presented data using forest plots where possible. For time‐to‐event data, we plotted (and, if appropriate, pooled) estimates of HRs and 95% CIs as presented in the study reports, using the generic inverse variance method in Review Manager 5 (RevMan 2020). Where time‐to‐healing was analysed as a continuous measure, but it was not clear if all wounds healed, we documented use of the outcome in the study, but did not summarise or use the data in any meta‐analysis.
We included only the relevant arms where a trial involves multiple arms. If two or more arms in comparison with control were eligible for the same meta‐analysis, we pooled data on the two or more arms and compared them with control.
Subgroup analysis and investigation of heterogeneity
When there appeared to be considerable between‐study heterogeneity we planned to explore the causes using the steps proposed by Cipriani 2013:
check the data extraction and data entry for errors and possible outlying studies;
if outliers existed, perform sensitivity analysis by removing them; and
if heterogeneity was still present, we planned to perform subgroup analyses/meta‐regression for study‐level characteristics (see below) in order to explain heterogeneity as much as possible (Thompson 1999).
For subgroup analysis/meta‐regression, we considered four study‐level characteristics: funding sources (binary: not‐for‐profit versus other/unclear); overall risk of bias (binary: low and unclear risk of bias versus high risk of bias); study designs (binary: parallel versus other designs); and follow‐up duration (continuous). However, none of our meta‐analyses or syntheses included more than 10 studies for a feasible subgroup analysis, so we did not undertake subgroup analysis by any of these factors.
Sensitivity analysis
For pooled analyses, where possible, we undertook sensitivity analyses to explore the impact of the following:
assuming participants with missing data had a healed leg ulcer (i.e. they were included in both the numerator and the denominator) followed by the analysis with the assumption that participants with missing data had unhealed leg ulcers;
removing unpublished data (i.e. abstracts and dissertations) from the analysis;
changing effects model (i.e. using random‐effects model for the main analysis, followed by a repeated analysis with a fixed‐effect model); and
removing clustered data from the analysis.
Summary of findings and assessment of the certainty of the evidence
We presented the main results of the review in summary‐of‐findings tables. These tables present key information about the certainty of the evidence, the magnitude of the effects of the interventions examined and the sum of available data for the main outcomes (Schünemann 2021).
We present the following outcomes in the summary‐of‐findings tables:
time to complete wound healing when analysed using appropriate survival analysis methods;
proportion of wounds completely healed during the trial period;
all reported adverse events;
participant health‐related quality of life/health status;
cost effectiveness;
mean pain score (Differences between protocol and review).
We used the principle of the GRADE approach to assess the certainty of the body of evidence associated with all outcomes (see Quality of the evidence). The GRADE approach defines the certainty of a body of evidence for the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The assessment of the certainty of a body of evidence using the GRADE approach involves consideration of within‐trial risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias (Schünemann 2021). The certainty of evidence can be assessed as being high, moderate, low or very low; RCT evidence has the potential to be high certainty.
When making decisions about methodological quality, we downgraded our assessment of the certainty of the evidence only when studies were classed as being at an overall high risk of bias. We did not downgrade for assessments of unclear overall risk of bias unless an outcome finding had unclear risk of bias in all domains, where we considered it as being at high overall risk of bias.
In assessing the precision of effect estimates we followed GRADE guidance using the combination of optimal information size (OIS), and the 95% CIs of effect estimates:
if the OIS criterion was not met, downgraded for imprecision, unless the sample size was very large (at least 2000, and perhaps 4000, participants);
if the OIS criterion was met and the 95% CI excluded no effect (i.e. the CI around the RR excludes 1.0), did not downgrade for imprecision; and
if the OIS criterion was met, and the 95% CI overlapped no effect (i.e. CI includes RR of 1.0) downgraded for imprecision if the CI failed to exclude important benefit or important harm (i.e. the 95% CIs included a relative risk reduction or increase of 25% or more).
For binary outcomes, we calculated the OIS on the basis of a relative risk reduction or increase of between 20% and 30%, as outlined in the GRADE Handbook and summarised below.
Time to wound healing: OIS = 524 participants for a reduction in hazard of time to healing of 25% (with 100 days' recruitment and 100 days' follow‐up: 80% power; alpha 5% and median time to healing in control group of 90 days).
Proportion of wounds healed: OIS = 308 participants for an increased relative risk of wound healing of 25% (80% power; alpha 5%; proportion healed in control group = 45.08%).
All reported adverse events: OIS = 295 participants for a decreased relative risk of adverse events of 25% (80% power; alpha 5%; proportion reported adverse events in control group = 45%).
For continuous outcomes, we used the rule‐of‐thumb threshold (OIS = 400) suggested by Schünemann 2013.
We considered downgrading twice for imprecision when, in addition to the rules above, the number of outcome events was considered to be low.
When assessing the remaining domains, we followed GRADE guidance (Schünemann 2013). Where data were not pooled we presented GRADE assessments for the above outcomes narratively in a summary‐of‐findings table (Murad 2017).
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; and Characteristics of studies awaiting classification.
Results of the search
The electronic searches identified 2052 records, including 1849 from electronic databases and 203 from trial registries. We excluded 139 duplicate records and screened 1913 records, of which we identified 252 as potentially eligible and obtained them in full‐text. Following full‐text screening we considered 31 records of 13 studies to be eligible for inclusion in this review (Cardoso 2019; Charles 1991; Daróczy 2006; Eriksson 1984a; Kikta 1988; Morrell 1998; O'Brien 2003; Rubin 1990; Taradaj 2007; Taylor 1998; Wong 2008a; Wong 2008b; Wong 2012).
From other resources, we identified Groenewald 1984 by scanning the reference list of Kikta 1988.
In total we include 14 studies (with 32 publications) in this review, of which Wong 2008a and Wong 2008b were from the same doctoral thesis: Wong 2008a was a feasibility study and Wong 2008b was the associated full trial. See Figure 1.
1.
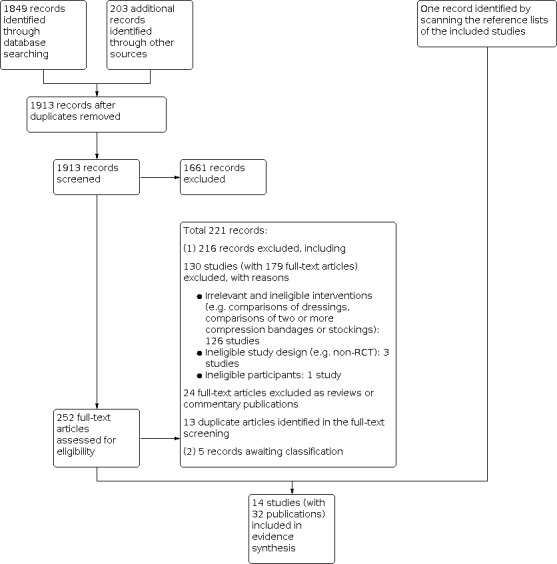
Study flow diagram.
Included studies
Types of studies
Of the 14 included studies (all RCTs) 13 had a parallel‐group design (Charles 1991; Daróczy 2006; Eriksson 1984a; Groenewald 1984; Kikta 1988; Morrell 1998; O'Brien 2003; Rubin 1990; Taradaj 2007; Taylor 1998; Wong 2008a; Wong 2008b; Wong 2012), and Cardoso 2019 applied a cross‐over design.
Ten of the 14 studies used individual participants as the unit of randomisation and analysis (Daróczy 2006; Eriksson 1984a; Groenewald 1984; Morrell 1998; O'Brien 2003; Rubin 1990; Taylor 1998; Wong 2008a; Wong 2008b; Wong 2012); one appeared to use legs as the unit of randomisation (i.e. randomising legs affected by venous ulcers into different study arms) and analysed outcome data by ulcers (Cardoso 2019); and three appeared to have individuals as the unit of randomisation but ulcers as the unit of analysis (Charles 1991; Kikta 1988; Taradaj 2007).
Of the 14 studies, eight had two arms and six had three arms (Daróczy 2006; Eriksson 1984a; Taradaj 2007; Wong 2008a; Wong 2008b; Wong 2012), while two of these (Daróczy 2006; Taradaj 2007) had a third arm that was not relevant to this review.
Five of the 14 included studies (with 854 participants) were conducted at more than one research site (Kikta 1988; Morrell 1998; Rubin 1990; Wong 2008b; Wong 2012). The included studies were conducted in: Brazil (Cardoso 2019), Hong Kong (Wong 2008a; Wong 2008b; Wong 2012), Hungary (Daróczy 2006), Ireland (O'Brien 2003), Poland (Taradaj 2007), Sweden (Eriksson 1984a), South Africa (Groenewald 1984), the UK (Charles 1991; Morrell 1998; Taylor 1998), and USA (Kikta 1988; Rubin 1990), most of which are high‐income and upper‐middle‐income economies.
In the 14 studies the median follow‐up duration was 12 weeks (range: one day to 12 months).
Types of participants
Age and sex at baseline
The 14 studies enrolled a total of 1391 participants with venous leg ulcers (median study sample size: 51 participants; range: 11 to 321). Across the eight studies that specified participant sex (Cardoso 2019; Morrell 1998; O'Brien 2003; Taradaj 2007; Taylor 1998; Wong 2008a; Wong 2008b; Wong 2012), 526 (50.1%) of participants were male and 524 (49.9%) were female. The average participant age was specified in 11 studies, with a median of 70.1 years (range: 58.0 to 76.5 years ) (Cardoso 2019; Charles 1991; Daróczy 2006; Eriksson 1984a; Morrell 1998; O'Brien 2003; Taradaj 2007; Taylor 1998; Wong 2008a; Wong 2008b; Wong 2012).
The aetiology of leg ulcers
Of the 14 studies, 12 (917 participants) described their participants as those with leg ulcers and with some markers of a venous aetiology, in terms of either a history or clinical evidence of chronic venous disease or a confirmed chronic venous insufficiency, or both (Cardoso 2019; Daróczy 2006; Kikta 1988; O'Brien 2003; Taradaj 2007; Wong 2008a; Wong 2008b; Wong 2012); having an ankle pressure/brachial pressure ratio (APBI) greater than 0.8 (Charles 1991; Morrell 1998; O'Brien 2003; Rubin 1990; Taylor 1998; Wong 2008a; Wong 2008b; Wong 2012) or 0.9 (Taradaj 2007). The two remaining studies described their participants as people with venous leg ulcers but did not specify the aetiology of leg ulcers or APBI value (Eriksson 1984a; Groenewald 1984).
Duration of leg ulcers and ulcer size at baseline
Of the 14 studies, nine reported the average duration of leg ulcers at baseline; the median was 22.0 months (range: 9.0 weeks to 31.6 months) (Charles 1991; Groenewald 1984; Kikta 1988; Morrell 1998; O'Brien 2003; Taradaj 2007; Wong 2008a; Wong 2008b; Wong 2012). Additionally, 11 of the 14 studies reported the average area size of leg ulcers at baseline: three studies (278 participants) having ulcers on average smaller than 5 cm2 (Daróczy 2006; O'Brien 2003; Taylor 1998); four studies (615 participants) having ulcers between 5 and 10 cm2 (Kikta 1988; Wong 2008a; Wong 2008b; Wong 2012); two studies (286 participants) having ulcers between 10 and 20 cm2 (Charles 1991; Morrell 1998); and two studies (85 participants) with ulcers larger than 20 cm2 (Rubin 1990; Taradaj 2007).
Care settings
Care settings were specified for 10 studies: two recruited participants from hospitals (Cardoso 2019; Taradaj 2007); one from an outpatient setting (Groenewald 1984); and seven from community settings (Charles 1991; Morrell 1998; O'Brien 2003; Taylor 1998; Wong 2008a; Wong 2008b; Wong 2012).
Types of interventions
Compression bandages or stockings (including the duration of applying compression and the frequency of changes) and comparators evaluated in the 14 studies are listed in Table 2.
1. Details of compression bandages or stockings and comparators applied.
| Study ID | Compression bandages or stockings | No compression | Comment |
| Compression unspecified | |||
| Daróczy 2006 | Undefined compression plus local povidone‐iodine (Betadine) | Local povidone‐iodine (Betadine) | ‐ |
| Short‐stretch bandages | |||
| Charles 1991 | Short‐stretch compression bandages (Rosidal, with the spiral technique and 1 ‐ 3 times of changes per week) that was expected to achieve mean pressure of 33 mmHg | Usual care, without further details | ‐ |
| Taradaj 2007 | Elastic short‐stretch bandages (Sigvaris) plus unspecified pharmacotherapy
|
Unspecified pharmacotherapy | Taradaj 2007 applied compression bandages and the control treatments only after removing affected veins on legs via operations in participants with venous leg ulcers. |
| Wong 2008a | Short‐stretch bandage (Rosidal)
|
A variety of dressings | ‐ |
| Wong 2008b | Short‐stretch bandage (Rosidal)
|
A variety of dressings | ‐ |
| Wong 2012 | Short‐stretch bandage (Rosidal)
|
A variety of dressings | ‐ |
| Four‐layer bandage | |||
| Morrell 1998 | Four‐layer bandage following the Charing Cross bandaging technique (with a weekly treatment)
|
A wide variety of treatments
|
In the comparator, 53% of 3433 visits at home used some form of compression treatment but not the same compression as the intervention group (4‐layer compression). |
| O'Brien 2003 | Four‐layer compression bandage (with a natural padding bandage, a light conformable bandage, a light compression bandage and a flexible cohesive bandage)
|
A variety of dressings | O'Brien 2003 stated that 5 participants in the control had compression applied at some stage during 3‐months interval. |
| Taylor 1998 | Four‐layer bandage following the Charing Cross bandaging technique (with a weekly treatment)
|
A wide variety of treatments applied without restriction other than the use of high‐compression bandaging | ‐ |
| Wong 2008a | Four‐layer bandage (Profore)
|
A variety of dressings | ‐ |
| Wong 2008b | Four‐layer bandage (Profore)
|
A variety of dressings | ‐ |
| Wong 2012 | Four‐layer bandage (Profore)
|
A variety of dressings | ‐ |
| Unna's boot | |||
| Cardoso 2019 | Unna's boot
|
Dressings unspecified | ‐ |
| Eriksson 1984a | Unna's boot
|
|
Based on descriptions of compression therapies, the review authors considered the compression used was Unna's boots. Eriksson 1984a replaced porcine skin dressings ‐ due to its unavailability ‐ with double‐layer bandage (i.e. Unna's boot) during the study period. |
| Groenewald 1984 | Unna's boot
|
Hydrocolloid dressing | Based on descriptions of compression therapies, the review authors considered the compression used was Unna's boots. |
| Kikta 1988 | Unna's boot
|
Hydroactive dressing (DuoDERM) | ‐ |
| Rubin 1990 | Unna's boot
|
Polyurethane foam dressing | ‐ |
A variety of compression bandages or stockings was evaluated in the 14 included studies, including elastic short‐stretch bandages (five studies; Charles 1991; Taradaj 2007; Wong 2008a; Wong 2008b; Wong 2012); four‐layer bandages including the Charing Cross bandaging technique (six studies; Morrell 1998; Taylor 1998; O'Brien 2003; Wong 2008a; Wong 2008b; Wong 2012); and Unna's boot (five studies; Cardoso 2019; Eriksson 1984a; Groenewald 1984; Kikta 1988; Rubin 1990). One study (Daróczy 2006) did not specify the type of compression therapies used. The sub‐bandage resting pressure applied was specified in seven studies (854 participants; Cardoso 2019; Charles 1991; O'Brien 2003; Taradaj 2007; Wong 2008a; Wong 2008b; Wong 2012) with a minimum of 18 mmHg and a maximum of 50 mmHg, whilst other studies (537 participants) did not specify the pressure level.
A wide range of treatments was described as comparators, including medicines (two studies; Daróczy 2006; Taradaj 2007), usual care received from district nurses (three studies; Charles 1991; Morrell 1998; Taylor 1998); and dressings (nine studies; Cardoso 2019; Eriksson 1984a; Groenewald 1984; Kikta 1988; O'Brien 2003; Rubin 1990; Wong 2008a; Wong 2008b; Wong 2012). Of the 14 studies, 10 (878 participants) did not specify the use of compression bandages or stockings for participants in comparators arms; three studies stated that their comparators did not preclude compression bandages or stockings (469 participants; Morrell 1998; O'Brien 2003; Taylor 1998) and one (44 participants; Eriksson 1984a) replaced one control arm with double‐layer bandage during the study period as the treatments used as the comparator were unavailable.
Seven studies specified co‐interventions they applied (e.g. specific dressings) (Charles 1991; Groenewald 1984; Kikta 1988; Rubin 1990; Taradaj 2007; Taylor 1998; Wong 2012), all stated or indicated that the same co‐interventions were applied in all study groups.
Source of funding
Of the 14 included studies, six specified the sources of funding, including: Morrell 1998 funded by a public health authority; O'Brien 2003 and Taylor 1998 financially supported by the producers of compression devices; Wong 2008a and Wong 2008b funded by a university; and Wong 2012 funded by both a public authority and device companies.
Excluded studies
We excluded 130 studies (with 179 records). The main reasons for exclusions were: irrelevant and ineligible interventions (e.g. comparisons of dressings, comparisons of two or more compression bandages or stockings; 126 studies); ineligible study design (e.g. non‐RCT; 3 studies); and ineligible participants (one study). We also identified 13 duplicates in screening full texts (see Figure 1).
Ongoing studies
We did not identify any ongoing studies.
Studies awaiting classification
We identified five studies (five records) that we could not classify as being eligible or not, as we were unable to obtain the full‐text versions despite extensive efforts, in part due to more limited access to intra‐library loans during the COVID‐19 period (Cherry 1990; Jünger 2008; Kuznetsov 2009; Robinson 1988; Stacey 2000).
Risk of bias in included studies
We summarise risk‐of‐bias assessments for the primary outcome of this review in Figure 2 and Figure 3.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
We judged four of the 14 studies to have an unclear overall risk of bias for the primary outcome (Cardoso 2019; Morrell 1998; O'Brien 2003; Rubin 1990). We judged the remaining 10 studies as having findings at high overall risk of bias.
Allocation
Of the 14 studies, four used appropriate methods to generate the random sequence and were judged to have low risk of selection bias (O'Brien 2003; Taylor 1998; Wong 2008b; Wong 2012). The remaining 10 studies did not adequately describe the randomisation methods.
Of the 14 studies, only Morrell 1998 was judged to have low risk of selection bias due to allocation concealment, because serially‐numbered, sealed, opaque allocation envelopes were used to adequately conceal allocation. The remaining 13 studies had an unclear risk of bias judgement due to the lack of relevant information.
Blinding
Five of the 14 studies were judged as being at high risk of performance bias for the leg‐ulcer healing outcome because they clearly stated that the blinding of participants and personnel was difficult to implement or was not implemented (Eriksson 1984a; Groenewald 1984; Wong 2008a; Wong 2008b; Wong 2012); and it was unclear if attempts were made to mitigate the risk. The remaining nine studies did not give sufficient information for judging if their risk of performance bias was high or low.
We judged six of the 14 studies to have low risk of detection bias for leg‐ulcer healing outcome (Eriksson 1984a; Groenewald 1984; O'Brien 2003; Taylor 1998; Wong 2008b; Wong 2012): all six studies applied devices to measure leg ulcer areas reliably, or involved independent outcome assessors for outcome measurement, or both. The remaining eight studies did not give sufficient information for judging if their risk of detection bias was high or low.
Incomplete outcome data
Of the 14 studies, Taylor 1998 was judged to have high risk of attrition bias for the leg‐ulcer healing outcome because there was a high proportion of dropouts and intention‐to‐treat (ITT) analysis was not performed. We rated eight studies at low risk of attrition bias (Daróczy 2006; Kikta 1988; Morrell 1998; O'Brien 2003; Rubin 1990; Wong 2008a; Wong 2008b; Wong 2012): all had low attrition rates (or no attrition), or ITT analysis was performed, or both. The remaining five studies had an unclear risk of bias judgement.
Selective reporting
We judged four of the 14 studies to be at high risk of reporting bias (Daróczy 2006; Kikta 1988; Wong 2008a; Wong 2012). The feasibility study by Wong 2008a measured multiple outcomes but only reported week‐12 ulcer‐healing outcome data. There were two publications for the Wong 2012 study, with one retracted by the corresponding journal; but the retracted publication appeared to contain a specific outcome that was not included in the unretracted publication. Charles 1991 and Morrell 1998 were judged as being at unclear risk of reporting bias and the remaining eight studies appeared to be free of this bias.
Other potential sources of bias
Of the 14 studies, we rated three at high risk of other bias because they used individuals as the unit of randomisation but leg ulcers as the unit of analysis (Charles 1991; Kikta 1988; Taradaj 2007). We judged Wong 2008a to be at unclear risk of other bias. We judged all the remaining studies to be free of other bias.
Effects of interventions
See: Table 1
See Table 1.
Unless otherwise stated we used a random‐effects analysis throughout; each pooled result presented is an average effect, rather than a common effect, and should be interpreted as such.
Comparison 1: Compression bandages or stockings compared with no compression (14 studies, 1391 participants)
All 14 studies assessed this comparison, of which Cardoso 2019 did not report analysable data for any outcomes.
Primary outcomes
Time‐to‐complete wound healing (follow‐up period one day to 12 months)
Seven studies (1096 participants) reported this outcome. See Appendix 3 for the outcome data. We were unable to collect analysable data from Daróczy 2006 and Taylor 1998. We pooled time‐to‐event data from five studies (733 participants: Kikta 1988; Morrell 1998; O'Brien 2003; Taylor 1998; Wong 2008b). Note that Wong 2008b reported a multivariable analysis adjusted for covariates (age, initial ulcer size, and ulcer duration). Kikta 1988 contained clustered data (participants randomised but outcome data reported on multiple ulcers for some participants); as the number of ulcers (87 ulcers) was close to the number of participants (n = 84) ulcer‐level data were included here. The pooled hazard ratio (HR) for healing is 2.17 (95% confidence interval (CI) 1.52 to 3.10; I2 = 59%; Analysis 1.1).
1.1. Analysis.

Comparison 1: Compression bandages or stockings compared with no compression, Outcome 1: Time‐to‐complete wound healing
Moderate‐certainty evidence suggests there is probably a shorter time to complete healing of venous leg ulcers in people wearing compression bandages or stockings compared with those not wearing compression. We downgraded the evidence across the five studies once for risk of bias (one study with clustered data and another small study were at high overall risk of bias in domains other than performance bias, and the other three studies with most of the data in this synthesis were at unclear overall risk of bias).
Subgroup analysis
As noted above, these studies are heterogeneous in terms of unit of analysis, care setting, follow‐up durations, risk of bias, and compression therapies applied. However, we did not perform any prespecified subgroup analysis because there are fewer than 10 studies.
Sensitivity analyses
Sensitivity analysis of removing unpublished data (i.e. abstracts and dissertations) . When the doctoral thesis Wong 2008b was removed from the data analysis, the evidence remained consistent with the main analysis (Appendix 4).
Sensitivity analysis with fixed‐effect rather than random‐effects model . The use of a fixed‐effect model resulted in no change in effect estimates. The evidence remained consistent with the main analysis (Appendix 4).
Post hoc sensitivity analysis of removing clustered data. When Kikta 1988 was removed, the result remained consistent with the main analysis (Appendix 4).
Proportion of wounds completely healed (follow‐up of one day to 12 months)
Ten studies (1215 participants) reported this outcome (Charles 1991; Daróczy 2006; Kikta 1988; Morrell 1998; O'Brien 2003; Rubin 1990; Taylor 1998; Wong 2008a; Wong 2008b; Wong 2012). See Appendix 3 for the outcome data.
Eight of the 10 studies reported analysable data: seven studies (1036 participants) (Morrell 1998; O'Brien 2003; Rubin 1990; Taylor 1998; Wong 2008a; Wong 2008b; Wong 2012) reported data by participant whilst Kikta 1988 reported clustered data (84 participants with 87 ulcers) but ulcer‐level data were included here, as the number of participants approximately equals the number of ulcers. The pooled risk ratio (RR) is 1.77 (95% CI 1.41 to 2.21; I2 = 65%; Analysis 1.2).
1.2. Analysis.

Comparison 1: Compression bandages or stockings compared with no compression, Outcome 2: Proportion of wounds completely healed during follow‐up
Of the remaining two studies (95 participants) without analysable data, Charles 1991 reported that 71% of leg ulcers completely healed in short‐stretch bandages and 25% in usual care. Daróczy 2006 reported that 82% of 21 participants with ulcers healed when using compression plus local povidone‐iodine (Betadine) and 62% of 21 participants with ulcers healed when using local povidone‐iodine (Betadine).
Across the 10 studies, there is moderate‐certainty evidence that people treated with compression bandages or stockings probably have more completely‐healed venous leg ulcers during follow‐up to 12 months than people not using compression. We downgraded the certainty of the evidence once for risk of bias (of the 10 studies, six with 569/1215 (46.8%) participants were at high risk of bias in the domains other than performance bias and the other four were at unclear risk of bias in some but not all domains).
Subgroup analysis
The studies are heterogeneous in terms of unit of analysis, care setting, follow‐up duration, risk of bias, and compression therapies applied and there was some statistical heterogeneity (Chi2 test P value = 0.006; Tau2 = 0.06; I2 = 65%). As noted in Subgroup analysis and investigation of heterogeneity, we removed an extreme value (Morrell 1998) and found that once it was removed, I2 went from 65% to 0% (Chi2 test P value = 0.51; Tau2 = 0.00; I2 = 0%) but the pooled RR of 1.94 (95% CI 1.67 to 2.26) remained consistent with Analysis 1.2 and still favoured the use of compression bandages or stockings. Morrell 1998 is notably different from the other studies, in that 53% of 3433 participant visits in the control arm involved provision of compression whilst the control arms of other studies either did not deliver compression or only delivered for a small proportion of participants. We did not perform any prespecified subgroup analysis because there are fewer than 10 included studies in Analysis 1.2.
Sensitivity analyses
Sensitivity analysis of considering participants with missing data as having unhealed leg ulcers . Four of the eight studies in Analysis 1.2 had missing data (Kikta 1988; Taylor 1998; Wong 2008a; Wong 2008b). The main analysis was not sensitive to considering participants with missing data as having unhealed ulcers (Appendix 4).
Sensitivity analysis of removing unpublished data (i.e. abstracts and dissertations) . The main analysis was not sensitive to the removal of the doctoral theses Wong 2008a; Wong 2008b from Analysis 1.2 (Appendix 4).
Sensitivity analysis with a fixed‐effect rather than random‐effects model . The main analysis was not sensitive to application of a fixed‐effect model (Appendix 4).
Post hoc sensitivity analysis of removing clustered data. When Kikta 1988 was removed, the result remained consistent with the main analysis (Appendix 4).
Adverse events (follow‐up period 8 weeks to 12 months)
Ten studies reported adverse events (Eriksson 1984a; Groenewald 1984; Kikta 1988; Morrell 1998; O'Brien 2003; Rubin 1990; Taylor 1998; Wong 2008a; Wong 2008b; Wong 2012). See Table 3. However, only three studies appeared to collect adverse event data following a prespecified method: two studies (501 participants) reported data by participant (Wong 2008b; Wong 2012) whilst Kikta 1988 reported clustered data (84 participants with 87 ulcers) and ulcer‐level data were included here, as the number of participants approximately equals the number of ulcers. The pooled RR is 0.98 (95% CI 0.25 to 3.80; I2 = 74%; Analysis 1.3). A post hoc sensitivity analysis of removing Kikta 1988 that reported clustered data resulted in a pooled RR of 1.60 (95% CI 0.74 to 3.43; I2 = 40%).
2. Results for adverse events reported in the included studies.
| Study ID | Adverse events in compression bandages or stockings | Adverse events in no compression | Inference |
| Eriksson 1984a | No case abandoned; No symptoms of peripheral arterial circulatory insufficiency |
6 participants stopped using Metallina(R) aluminium foil dressing earlier because of poor effect (ulcer size increase or infections, or both) | Not relevant |
| Groenewald 1984 | 6 of 36 withdrew due to treatment changes required (overwhelming sepsis and ulcer size increase) | 7 of 36 withdrew in hydrocolloid dressing due to non‐compliance (n = 2) and treatment stopped (n = 5 including 2 having pain and irritation and 3 with overwhelming sepsis); Few unfavourable effects recorded for those using hydrocolloid dressings; An increased tendency toward fungus infections noted in some cases treated with hydrocolloid dressings |
Not relevant |
| Kikta 1988 | 0 of 30 (0%) in Unna’s boot | 10 of 39 ulcers (26%) in hydroactive dressing including 8 with a reddish‐green exudate; 1 with cellulitis; and 1 with circumferential ulcers and cellulitis | Fisher’s exact test P‐value = 0.004 |
| Morrell 1998 | 9 deaths of 120 participants using 4‐layer bandages | 7 of 113 using usual care | Not relevant |
| O'Brien 2003 | 1 death in 4‐layer bandage | Information not reported | Not relevant |
| Rubin 1990 | No wound complications necessitating hospital admission of cessation of therapy | No wound complications necessitating hospital admission of cessation of therapy; 9 of 17 participants withdrew due to wound odour |
Not relevant |
| Taylor 1998 | 1 death, and 1 postponed to apply compression due to scabies | 1 death; and 1 developed cellulitis in conventional care | Not relevant |
| Wong 2008a | See Inference | See Inference | Not relevant; 1 was reported having wound infection at week 4, and 1 with ankle movement restriction. However, the study authors did not specify which group these participants were from |
| Wong 2008b | Short‐stretch bandages: 8 of 60 4‐layer bandages: 13 of 60 |
Usual care without compression: 4 of 60 | Not relevant |
| Wong 2012 | 4‐layer compression bandages: 16 of 107; Short‐stretch bandages: 10 of 107 |
Usual care without compression: 11 of 107 | Not relevant |
Multiple publications of the same study (Wong 2012) reported different results of adverse events. The result used in this table was from the Table 5 of the study report published in the Journal of the European Academy of Dermatology and Venereology.
1.3. Analysis.

Comparison 1: Compression bandages or stockings compared with no compression, Outcome 3: Adverse events
It is uncertain whether there is any difference in the risk of adverse events associated with using compression and not using compression. Evidence is of very low certainty, downgraded once for risk of bias (two studies with the larger numbers of participants were at high risk of bias in some domains and one study contributing 30.6% of the weight was at unclear risk of bias), twice for substantial inconsistency, as the clustered data were inconsistent with the data reported by participant, and once for imprecision because the CIs appeared to include the possibility of both benefit and harm as well as no effect.
Secondary outcomes
Participant health‐related quality of life/health status (follow‐up period 12 weeks to 12 months)
Five studies (964 participants) reported this outcome and the outcome measurements varied between studies (Morrell 1998; O'Brien 2003; Wong 2008a; Wong 2008b; Wong 2012). We synthesised evidence from all these studies, except for Wong 2008a which did not present the endpoint outcome data.
All data reported in these studies are summarised in Table 4. We pooled data from two studies (426 participants with available data; Wong 2008b; Wong 2012; Analysis 1.4). The MD in the total score of the Charing Cross Venous Ulcer Questionnaire (lower scores = better quality of life) is −6.87 (95% CI −13.10 to −0.64) between using compression bandages or stockings and no compression, which favours the use of compression bandages or stockings. However, Analysis 1.4 showed no difference in the physical component, mental component, and functional status of the SF‐12 (higher scores = better quality of life).
3. Results of participant health‐related quality of life/health status reported in the included studies.
| Study ID | Questionnaires | Domains | Compression | Dressings | Inference |
| Morrell 1998 |
|
Not relevant | Data at 12 weeks and 12 months were not presented | Data at 12 weeks and 12 months were not presented | The study authors stated that "for most dimensions of the SF‐36 and EuroQol, health status deteriorated over time, with no difference between the groups" |
| O'Brien 2003 | SF‐36 (higher score = better health) | Physical function | Median 70 (IQR 45 to 85) at 6 weeks (n = 79) | Median 50 (IQR 25 to 80) at 6 weeks (n = 91) | Mann‐Whitney U test P = 0.001 |
| Role‐physical | 100 (0 to 100) | 25 (0 to 100) | P = 0.006 | ||
| Bodily pain | 84 (61 to 100) | 72 (51 to 100) | P = 0.840 | ||
| General health | 77 (62 to 87) | 72 (62 to 82) | P = 0.202 | ||
| Vitality | 75 (60 to 80) | 60 (55 to 75) | P = 0.160 | ||
| Social function | 100 (75 to 100) | 87.5 (62.5 to 100) | P = 0.322 | ||
| Role‐emotional | 100 (100 to 100) | 100 (33.3 to 100) | P = 0.150 | ||
| Mental health | 88 (80 to 92) | 88 (76 to 92) | P = 0.030 | ||
| Disease‐specific quality of life instrument for chronic lower limb venous insufficiency (CIVIQ) (lower score = better status) | Pain | Median 18.8 (IQR 6.3 to 37.5) at 6 weeks (n = 79) | Median 31.3 (IQR 18.8 to 43.8) at 6 weeks (n = 91) | P = 0.140 | |
| Physical | 12.5 (6.3 to 37.5) | 37.5 (12.5 to 62.5) | P = 0.006 | ||
| Social | 33.3 (16.7 to 41.7) | 41.7 (25 to 58.3) | P = 0.001 | ||
| Psychological | 13.9 (11.1 to 25) | 19.4 (11.1 to 27.8) | P = 0.488 | ||
| Global | 18.8 (12.5 to 31.3) | 28.8 (18.8 to 43.8) | P = 0.006 | ||
| Wong 2008b | SF‐12 (higher score = better health) | Mental component | Short‐stretch bandages: mean 48.7 (SD 11.5) at 6 weeks; 47.2 (8.7) at 12 weeks Four‐layer bandages: 47.3 (9.7) at 6 weeks; 50.2 (7.3) at 12 weeks |
No compression: 47.6 (13.3) at 6 weeks; 47.7 (12.8) at 12 weeks |
‐ |
| Physical component | Short‐stretch bandages: mean 40.8 (SD 11.2) at 6 weeks; 47.0 (SD 11.5) at 12 weeks; Four‐layer bandages: 41.3 (10.0) at 6 weeks; 45.2 (11.9) at 12 weeks |
No compression: 40.7 (10.7) at 6 weeks; 41.3 (11.3) at 12 weeks |
‐ | ||
| Charing Cross Venous Ulcer Questionnaire (lower score = better health) | Total scores | Short‐stretch bandages: mean 48.8 (SD 12.6) at 6 weeks; 34.5 (17.2) at 12 weeks; Four‐layer bandages: 49.1 (13.0) at 6 weeks; 36.4 (19.0) at 12 weeks |
No compression: 46.7 (14.1) at 6 weeks; 46.0 (20.6) at 12 weeks | ‐ | |
| Wong 2012 | SF‐12 (higher score = better health) | Functional status | Short‐stretch bandages: mean 40.5 (SD 7.31) at 12 weeks; 40.7 (7.15) at 24 weeks; Four‐layer bandages: 38.4 (9.19) at 12 weeks; 38.6 (9.38) at 24 weeks |
Dressings: mean 40.8 (SD 7.51) at 12 weeks; 40.3 (7.57) at 24 weeks |
RMANOVA test for pre‐ and post‐treatment scores at 12 weeks: P < 0.003 for short‐stretch bandages; P < 0.007 for 4‐layer bandages; P = 0.060 for dressings Inference pre‐ vs post‐treatment scores at 24 weeks not presented |
| Mental component | Short‐stretch bandages: mean 47.3 (SD 8.82) at 12 weeks; 55.3 (8.58) at 24 weeks; Four‐layer bandages: 50.0 (8.17) at 12 weeks; 55.2 (8.48) at 24 weeks |
Dressings: mean 47.2 (SD 12.4) at 12 weeks; 56.5 (8.30) at 24 weeks |
Pre‐ and post‐treatment scores at 12 weeks: all 3 groups P < 0.001 At 24 weeks: all P < 0.001 |
||
| Physical component | Short‐stretch bandages: mean 47.5 (SD 11.1) at 12 weeks; 53.5 (9.37) at 24 weeks; Four‐layer bandages: 47.7 (10.9) at 12 weeks; 54.0 (9.12) at 24 weeks |
Dressings: mean 44.1 (SD 11.8) at 12 weeks; 53.1 (9.68) at 24 weeks |
Pre‐ and post‐treatment scores at 12 weeks: all 3 groups P ≤ 0.001 At 24 weeks: all P < 0.001 |
||
| Charing Cross Venous Ulcer Questionnaire (lower score = better health) | Total scores | Short‐stretch bandages: mean 21.6 (SD 16.4) at 12 weeks; 21.0 (15.8) at 24 weeks; Four‐layer bandages: 22.4 (16.5) at 12 weeks; 20.9 (15.2) at 24 weeks |
Dressings: mean 25.1 (SD 18.9) at 12 weeks; 25.1 (18.1) at 24 weeks |
Pre‐ and post‐treatment scores at 12 weeks: short‐stretch bandages and 4‐layer bandages: P < 0.001. Dressing P = 0.047 At 24 weeks: short‐stretch bandages and four‐layer bandages P < 0.035. Dressing not significant |
1.4. Analysis.

Comparison 1: Compression bandages or stockings compared with no compression, Outcome 4: Participant health‐related quality of life/health status
Of the two studies that had no analysable data, Morrell 1998 (233 participants) stated that, for most dimensions of the SF‐36 and EuroQol, health status deteriorated over time but was not different between four‐layer bandages and usual care. O'Brien 2003 (200 participants) reported a statistical difference, favouring the use of compression bandages or stockings, in some dimensions of the SF‐36 (including physical function, role‐physical, mental health) and the disease‐specific quality of life instrument for chronic lower limb venous insufficiency (CIVIQ) (physical, social, and global dimensions), but not in others.
Overall, low‐certainty evidence suggests that compression bandages or stockings may improve participant health‐related quality of life in some (but not all) aspects during the follow‐up of 12 weeks to 12 months in comparison with no compression. We downgraded the certainty of the evidence twice for substantial inconsistency due to the variation in the reported results, particularly for health status.
Cost effectiveness (follow‐up period 12 weeks and 12 months)
Three studies (486 participants) reported this outcome (Charles 1991; Morrell 1998; O'Brien 2003), but we were unable to pool their data.
Without reporting any data, Charles 1991 (53 participants) noted that the short‐stretch bandage was more cost‐effective than usual care, as it could be washed and reused repeatedly. Morrell 1998 (233 participants) reported an incremental cost‐effectiveness ratio of GBP 2.46 (95% CI −31.94 to 99.12) per ulcer‐free week (1995 prices) associated with using the four‐layer bandage in leg‐ulcer clinics compared with no compression delivered outside the clinic setting. Using a cost analysis, O'Brien 2003 (200 participants) reported that the median cost per leg healed was significantly less for four‐layer bandages than for dressings (P = 0.04).
It is uncertain whether compression bandages or stockings are cost‐effective compared with no compression in wound healing. Evidence is of very low certainty, downgraded once for risk of bias (one small study was at high overall risk of bias in domains other than performance bias, and the other two studies were at unclear risk of bias in some domains), once for indirectness (the outcomes in Charles 1991 and O'Brien 2003 did not appear to be expressed as incremental mean cost per incremental gain in benefit), and once for inconsistency in cost‐effectiveness results between studies.
Mean pain score (median follow‐up period 12 weeks minimum 12 weeks maximum 12 months)
Six studies (1048 participants) reported this outcome, and varied in the way they measured pain (Kikta 1988; Morrell 1998; O'Brien 2003; Wong 2008a; Wong 2008b; Wong 2012). All data reported in the included studies are summarised in Table 5.
4. Results of mean pain scores reported in the included studies.
| Study ID | Pain measurement instruments | Compression bandages or stockings | No compression | Comments |
| Kikta 1988 | Not specified; using a scale with grades from 1 to 10 (1 = the least painful) | Mean 2.4 (SEM 0.4) in Unna’s boot | Mean 1.2 (SEM 0.1) in hydroactive dressing | Clustered data; Student’s t test P value 0.007 |
| Morrell 1998 | Leg ulcer pain using the short‐form McGill Pain Questionnaire (SF‐MPQ) | Data were not presented | Data were not presented | Participants treated with 4‐layer bandages were more likely to experience a reduction in leg ulcer pain per month than those using usual care |
| O'Brien 2003 | Pain measured using the domain of the quality of life questionnaire CIVIO (a lower score = less pain) | Median 18.8 (IQR 6.3 to 37.5) | Median 31.3 (IQR 18.8 to 43.8) | P value 0.140 |
| Wong 2008b | Brief Pain Inventory instrument with a 10‐point VAS scale (0 to 10; 0 = least painful) |
Mean 1.9 (SD 1.8) for short‐stretch bandages (n = 50) 2.1 (2.1) for 4‐layer bandages (n = 46)
1.1 (1.5) for short‐stretch bandages (n = 50) 1.2 (1.7) for 4‐layer bandages (n = 46) |
3.1 (2.5) for usual care (n = 54)
3.0 (2.8) for usual care (n = 54) |
‐ |
| Wong 2012 | Brief Pain Inventory instrument with a 10‐point VAS scale (0 to 10; 0 = least painful) |
Mean 1.25 (SD 1.84) in short‐stretch bandages (n = 95) 1.43 (1.77) in 4‐layer bandages (n = 87)
Mean 1.25 (SD 1.90) in short‐stretch bandages; 1.38 (1.87) in 4‐layer bandages |
2.61 (2.40) in usual care (n = 94)
2.56 (2.46) in usual care |
‐ |
IQR: interquartile range; SD: standard deviation; SEM: standard error of the mean; VAS: visual analogue scale
Of the six studies, Wong 2008a did not report endpoint outcome data. We pooled data from three studies with analysable data: two (426 participants having available data) reported data by participant (Wong 2008b; Wong 2012), whilst Kikta 1988 reported ulcer‐level (clustered) data (69 ulcers with available data) and the ulcer‐level data were included here. The analysis showed people using compression bandages or stockings had a lower mean pain score than those using no compression (i.e. dressings) (MD −1.39, 95% CI −1.79 to −0.98; Analysis 1.5). A post hoc sensitivity analysis removing Kikta 1988 that reported clustered data resulted in a pooled MD of −1.48 (95% CI −2.05 to −0.91; I2 = 29%).
1.5. Analysis.

Comparison 1: Compression bandages or stockings compared with no compression, Outcome 5: Mean pain score
Of the other two studies that did not report analysable data, Morrell 1998 (233 participants) stated that people treated with four‐layer bandages were more likely to experience a reduction in leg ulcer pain per month than those using usual care; and O'Brien 2003 (200 participants) reported a lower median of pain scores among those using four‐layer bandages than those using dressings (median 18.8, IQR 6.3 to 37.5; and 31.3, 18.8 to 43.8, respectively; P = 0.14).
Moderate‐certainty evidence suggests the use of compression probably reduces mean pain score compared with no compression. Across the five studies with data or results synthesised, we downgraded the evidence certainty once for risk of bias, as two studies with 345 participants were at high overall risk of bias.
Discussion
Summary of main results
We report a review of 14 RCTs on the effects of compression compared with no compression on the healing of venous leg ulcers. Compression bandages and stockings used in the included studies varied and some study reports did not adequately define the compression. The most frequently used systems were short‐stretch bandages, four‐layer compression and Unna's boot. The no‐compression comparators also varied across the included studies and included 'usual care' where no details were specified, pharmacological treatment, a variety of dressings, and a wide variety of treatments where some participants received compression (but it was not the norm).
Primary outcomes
There is moderate‐certainty evidence that there is probably a shorter time to complete healing of venous leg ulcers in people wearing compression bandages or stockings compared with those not wearing compression over a 12‐month follow‐up (five studies with 733 participants); and that people treated with compression bandages or stockings probably have more completely healed venous leg ulcers during follow‐up to 12 months than people not using compression (10 studies with 1215 participants). It is uncertain whether there is any difference in adverse effect rates between using compression bandages or stockings and no compression (three studies with 585 participants; very low‐certainty evidence).
Secondary outcomes
Moderate‐certainty evidence suggests that the use of compression bandages or stockings probably reduces pain compared with no compression (five studies with 859 participants and 69 ulcers in other participants).
There is low‐certainty evidence that compression bandages or stockings versus no compression may improve disease‐specific quality of life but not all aspects of general health status during the follow‐up of 12 weeks to 12 months (four studies with 859 participants).
There is uncertainty as to whether use of compression bandages or stockings is more cost‐effective than not using them (three studies with 486 participants).
Overall completeness and applicability of evidence
As a result of the extensive literature searches, we consider that this review covers all potential RCT evidence on compression bandages or stockings versus no compression for healing venous leg ulcers. However, there are limitations in the completeness and applicability of the evidence identified.
Whilst compression bandages and stockings are widely used, data on the effects of compression bandages or stockings on ulcer healing are sparse for the numbers of studies and participants, and the duration of follow‐up: 14 studies included in this review enrolled a total of 1391 participants, with a median study sample size of 51 participants (range: 11 to 321). Only four studies enrolled more than 100 participants and they together accounted for 67% (934/1391) of the participants; they were all from community settings that were most represented in the review. Three of these four studies were conducted in more than one research site. The duration of follow‐up was relatively short, with a median follow‐up duration of 12 weeks.
Participants included in the studies had average ages ranging from 58.0 to 76.5 years. The included studies all described their participants as having venous leg ulcers, but only 66% (917/1391) of participants had leg ulcers with a clearly‐specified aetiology (a history or clinical evidence of chronic venous disease, the confirmed cause of chronic venous insufficiency, or both), or had an APBI of greater than 0.8 or 0.9. The duration of leg ulcers amongst study participants had a median of 22.0 months (range: 9.0 weeks to 31.6 months). The average baseline area of leg ulcers amongst study participants also varied: three studies had participants with an average ulcer size of less than 5 cm2; four had ulcers between 5 and 10 cm2; two had ulcers between 10 and 20 cm2; and two had ulcers larger than 20 cm2.
Participants were recruited from community or outpatient settings and most were from high‐income and upper‐middle‐income economies.
Studies included in this review used a range of compression bandages or stockings, including elastic short‐stretch bandages (five studies); four‐layer bandage including the Charing Cross bandaging technique (six studies); and Unna's boot (five studies). There were no studies of systems such as inelastic paste systems, e.g. Setocrepe, or two‐component bandage systems e.g. 3M Coban 2 Compression System.
The magnitude of sub‐bandage resting pressure applied varied between studies: only seven included studies specified the pressure applied for 854 participants (minimum 18 mmHg, maximum 50 mmHg; i.e. moderate or high compression according to Thomas 1995).
Another limitation in the included studies was the variation in comparators applied, including medications (two studies), usual care from community nurses (three studies); and dressings (nine studies). Importantly, in three studies, participants in the no‐compression groups could receive compression (Morrell 1998; O'Brien 2003; Taylor 1998), and one small study (Eriksson 1984a) replaced the dressings comparator with compression bandages during the study period.
Whilst this review included a total of 14 studies, there is a very limited evidence base on adverse effects, participant health‐related quality of life, and cost effectiveness.
We note that we did not synthesise evidence on the outcome of participant adherence to compression treatment, as we initially planned, because this outcome was only relevant to the compression arm of these studies. Also, studies aiming to evaluate ulcer healing can only provide observational data for the ulcer recurrence outcome. We therefore did not consider evidence on ulcer recurrence as an outcome in this review, as we had initially planned.
Quality of the evidence
We assessed the certainty of evidence for all six outcomes using GRADE. We report our assessment results in Table 1, where we present the number of studies and the number of participants contributing to evidence synthesis. Evidence from two of the six syntheses was of very low certainty, one was of low certainty and three were of moderate certainty. Our downgrading was largely due to high overall risk of bias, and inconsistency across the studies.
Limitations in study design
We downgraded once for five of the six evidence syntheses for overall risk of bias. When judging overall risk of bias, we considered all domains. However, in assessing evidence certainty, we acknowledge that the blinding of participants and personnel is impractical for the comparison of compression bandages or stockings versus no compression and did not downgrade for this blinding. We judged that 10 of the 14 studies were at high overall risk of bias and seven of the 10 studies were at high risk of bias in domains other than blinding of participants and personnel.
Indirectness of evidence
We considered all the evidence to be direct (and so did not downgrade), except that two of the three studies included in cost‐effectiveness evidence synthesis appeared to report cost analyses rather than full cost‐effectiveness evaluations, which express cost effectiveness as incremental mean cost per incremental gain in benefit, or vice versa.
Inconsistency of results and unexplained heterogeneity
We downgraded for inconsistency for three evidence syntheses, largely because we found statistical heterogeneity in these syntheses or inconsistency of reported results between studies. The three outcomes this relates to are adverse events, health‐related quality of life, and cost effectiveness.
Note that none of these meta‐analyses or syntheses included more than 10 studies for a feasible subgroup analysis; so, despite the fact that we found heterogeneity by unit of analysis, overall risk of bias, care settings, follow‐up durations, or compression therapies applied between the included studies, we only considered the unit‐of‐analysis factor in our evidence syntheses, but did not consider other factors for subgroup analysis.
Imprecision of results
We only downgraded for imprecision for the adverse event synthesis, as the numbers of participants included in other meta‐analyses or syntheses were more than our estimated optimal information size (OIS). The downgrading for adverse events was due to the wide confidence intervals in the data analysis.
Publication bias
We did not downgrade for publication bias because firstly we have confidence in the comprehensiveness of our literature searches, and secondly we did not find any clear evidence of non‐reporting bias of study results. Although we planned to produce funnel plots for meta‐analysis to visually inspect for publication bias, there was no analysis including more than 10 studies.
Potential biases in the review process
We followed prespecified methods to review evidence in order to prevent potential bias in the review process. We ran comprehensive electronic searches, searched trial registries, and checked references of included studies and systematic reviews identified in electronic searches. We also contacted study authors of four studies (Cardoso 2019; Wong 2008a; Wong 2008b; Wong 2012) to clarify details. We included a study that had one retracted publication (Wong 2012).
This review also has limitations. Firstly, we did not consider the differences between specific compression bandages or stockings: four‐layer compression, Unna's boot and short‐stretch bandages were covered by the generic term of compression bandages or stockings. Similarly, the difference between specific comparators was not considered in this review. Because of these, heterogeneity between studies in this review was inevitable. However, because of the limited number of included studies, we did not undertake subgroup analysis by the types of specific compression bandages or stockings or specific comparators. Secondly, for all of the six outcomes in this review, the included studies measured outcomes differently, or reported outcome data in ways that could not be meta‐analysed. For example, of the seven studies which included data for the 'Time to complete wound healing' outcome, we were only able to pool data for five studies (Analysis 1.1). We therefore did not undertake or rely on any single meta‐analysis in summarising the findings and assessing the certainty of evidence. We reviewed all studies included for any outcome and assessed the certainty of evidence using the approach described in Murad 2017. Thirdly, in terms of the data on time to complete wound healing, we estimated the HRs and CIs for two studies included in Analysis 1.1 (Kikta 1988; Taylor 1998) using the methods described in Tierney 2007, whilst recognising that those calculated data (and the associated meta‐analysis) might be inaccurate. We noted that the time‐to‐event data analysis using the HRs and CIs we calculated appeared to agree with associated binary data analyses, as we expected.
Agreements and disagreements with other studies or reviews
Our electronic searches identified three systematic reviews or meta‐analyses (De Carvalho 2018; Mauck 2014; Weller 2012b) that were published following O'Meara 2012 (the review for which this is a major update).
Of these four reviews, only two had evidence for compression bandages or stockings versus no compression: O'Meara 2012 focused on the clinical effectiveness of using compression bandages or stockings versus no compression on venous leg‐ulcer healing, whilst Weller 2012b reviewed RCT‐based economic evaluations on this topic.
This review includes all relevant RCTs identified by O'Meara 2012 and Weller 2012b and added a further six studies. This review applied new Cochrane methodological requirements (e.g. the use of GRADE assessments) that were not used in O'Meara 2012 or Weller 2012b. As a result of the inclusion of more studies and applications of new methods, this review provides updated evidence, finding moderate‐certainty evidence that use of compression bandages or stockings results in a shorter complete wound‐healing time and a higher proportion of people having complete wound healing. This is consistent with O'Meara 2012, concluding that 'there is some evidence that venous ulcers heal more rapidly with compression than without.'
Pain is a common complication experienced by people affected by venous leg ulcers. Although application of compression may cause pain temporarily, ulcer‐related pain can be chronic. This review has identified new evidence that the use of compression bandages and stockings probably reduces pain compared with no compression.
Our review also found evidence, albeit uncertain, on the cost effectiveness of using compression bandages and stockings.
Authors' conclusions
Implications for practice.
People with venous leg ulcers who wear compression bandages or stockings probably experience wound healing more quickly, and more people are likely to experience complete ulcer healing than people who do not wear compression. This is moderate‐certainty evidence, meaning that the true effect is likely to be close to the reported estimate of the effect in this review. Risk of bias of some of the included studies resulted in downgrading of the certainty of evidence. The use of compression bandages or stockings probably reduces pain and may improve disease‐specific quality of life. However, uncertainty remains about the relative risks of adverse effects, and the cost effectiveness of compression bandages or stockings versus no compression.
Implications for research.
In many countries, compression bandages and stockings have become essential components of care for people with venous leg ulcers. Future research should focus on identifying the most effective and acceptable ways of delivering compression. Future studies should collect and analyse data on adverse events and cost effectiveness, and should explore whether certain modes of compression are associated with more patient adherence and greater acceptability.
Limitations in the existing evidence are due to studies with small sample sizes and suboptimal RCT designs. Given that the baseline prevalence of venous leg ulcers is not high and most people with this condition stay in community settings, future research can be conducted in multiple research sites and should carefully consider sample size calculations. Over‐estimation of event rates that fail to occur can lead to imprecision and less robust effect estimates.
Future studies should also consider carefully the choice of outcomes they report; time‐to‐event data for complete wound healing should be used in trials. Careful and consistent assessment and reporting of adverse events including pain score need to be undertaken. Further studies should aim to collect and report health‐related quality of life using validated measures and should include cost‐effectiveness analysis where possible. Participant adherence to compression bandages or stockings may be key to successful wound healing and should be considered in any future research.
Any future trials must be undertaken to the highest standard possible. Whilst it is challenging to avoid the risk of performance bias in trials of compression devices, where applicable, strictly implementing a standardised intervention plan can help to minimise risk. It is also important to ensure that protocols mandate balanced use of co‐interventions (e.g. dressings) across trial arms. Follow‐up periods should be for as long as possible, and clinically relevant in different settings.
History
Protocol first published: Issue 8, 2019
Acknowledgements
The authors are grateful to the following peer reviewers who provided feedback (including consumer peer review comments) on both the protocol and review: Elizabeth McInnes, Janet Gunderson and Una Adderley; and also to Clifford Richardson, who provided feedback on the protocol for this review. Thanks are also due to Jessica Sharp for copy‐editing the protocol and Kate Cahill for copy‐editing the review, and to Cochrane Muskuloskeletal, Oral, Skin and Sensory Network Editors Peter Tugwell and Jennifer Hilgart for feedback and final approval of the review for publication.
Appendices
Appendix 1. Search strategies
Cochrane Wounds Specialised Register
1 MESH DESCRIPTOR Leg Ulcer EXPLODE ALL AND INREGISTER
2 ((varicose next ulcer*) or (venous next ulcer*) or (leg next ulcer*) or (stasis next ulcer*) or (crural next ulcer*) or (ulcus next cruris) or (ulcer* next cruris)) AND INREGISTER
3 #1 OR #2
4 MESH DESCRIPTOR Compression Bandages EXPLODE ALL AND INREGISTER
5 compression* AND INREGISTER
6 stocking* or hosiery AND INREGISTER
7 sock or socks or tights AND INREGISTER
8 bandag* AND INREGISTER
9 wrapp* AND INREGISTER
10 #4 OR #5 OR #6 OR #7 OR #8 OR #9
11 #3 AND #10
The Cochrane Central Register of Controlled Clinical Trials (CENTRAL)
#1 MeSH descriptor: [Leg Ulcer] explode all trees
#2 ((varicose next ulcer*) or (venous next ulcer*) or (leg next ulcer*) or (stasis next ulcer*) or (crural next ulcer*) or (ulcus next cruris) or (ulcer* next cruris)):ti,ab,kw
#3 #1 or #2
#4 MeSH descriptor: [Compression Bandages] explode all trees
#5 compression*:ti,ab,kw
#6 stocking* or hosiery:ti,ab,kw
#7 sock or socks or tights:ti,ab,kw
#8 bandag*:ti,ab,kw
#9 wrapp*:ti,ab,kw
#10 #4 or #5 or #6 or #7 or #8 or #9
#11 #3 and #10 in Trials
Ovid MEDLINE
1 exp Leg Ulcer/
2 (varicose ulcer* or venous ulcer* or leg ulcer* or stasis ulcer* or (lower extremit* adj ulcer*) or crural ulcer* or ulcus cruris or ulcer* cruris).tw.
3 1 or 2
4 exp Compression Bandages/
5 compression*.tw.
6 (stocking* or hosiery).tw.
7 (sock or socks or tights).tw.
8 bandag*.tw.
9 wrapp*.tw.
10 or/4‐9
11 3 and 10
12 randomized controlled trial.pt.
13 controlled clinical trial.pt.
14 randomi?ed.ab.
15 placebo.ab.
16 clinical trials as topic.sh.
17 randomly.ab.
18 trial.ti.
19 or/12‐18
20 exp animals/ not humans.sh.
21 19 not 20
22 11 and 21
Ovid Embase
1 exp Leg Ulcer/
2 (varicose ulcer* or venous ulcer* or leg ulcer* or stasis ulcer* or (lower extremit* adj ulcer*) or crural ulcer* or ulcus cruris or ulcer* cruris).tw.
3 1 or 2
4 exp Compression Therapy/
5 exp Compression Bandage/
6 exp Compression Garment/
7 compression*.tw.
8 (stocking* or hosiery).tw.
9 (sock or socks or tights).tw.
10 bandag*.tw.
11 wrapp*.tw.
12 or/4‐11
13 3 and 12
14 Randomized controlled trial/
15 Controlled clinical study/
16 Random$.ti,ab.
17 randomization/
18 intermethod comparison/
19 placebo.ti,ab.
20 (compare or compared or comparison).ti.
21 ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab.
22 (open adj label).ti,ab.
23 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab.
24 double blind procedure/
25 parallel group$1.ti,ab.
26 (crossover or cross over).ti,ab.
27 ((assign$ or match or matched or allocation) adj5 (alternate or group$1 orintervention$1 or patient$1 or subject$1 or participant$1)).ti,ab.
28 (assigned or allocated).ti,ab.
29 (controlled adj7 (study or design or trial)).ti,ab.
30 (volunteer or volunteers).ti,ab.
31 trial.ti.
32 or/14‐31
33 (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.)
34 32 not 33
35 13 and 34
EBSCO CINAHL Plus
S37 S13 AND S36
S36 S35 NOT S34
S35 S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28
S34 S32 NOT S33
S33 MH (human)
S32 S29 OR S30 OR S31
S31 TI (animal model*)
S30 MH (animal studies)
S29 MH animals+
S28 AB (cluster W3 RCT)
S27 MH (crossover design) OR MH (comparative studies)
S26 AB (control W5 group)
S25 PT (randomized controlled trial)
S24 MH (placebos)
S23 MH (sample size) AND AB (assigned OR allocated OR control)
S22 TI (trial)
S21 AB (random*)
S20 TI (randomised OR randomized)
S19 MH cluster sample
S18 MH pretest‐posttest design
S17 MH random assignment
S16 MH single‐blind studies
S15 MH double‐blind studies
S14 MH randomized controlled trials
S13 S3 AND S12
S12 S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11
S11 TI wrapp* OR AB wrap
S10 TI bandag* OR AB bandag*
S9 TI ( sock or socks or tights ) OR AB ( sock or socks or tights )
S8 TI ( stocking* or hosiery ) OR AB ( stocking* or hosiery )
S7 TI compression* OR AB compression*
S6 (MH "Elastic Bandages")
S5 (MH "Compression Therapy")
S4 (MH "Compression Garments")
S3 S1 OR S2
S2 TI ( ((varicose ulcer*) or (venous ulcer*) or (leg ulcer*) or (stasis ulcer*) or (crural ulcer*) or (ulcus cruris) or (ulcer* cruris)) ) OR AB ( ((varicose ulcer*) or (venous ulcer*) or (leg ulcer*) or (stasis ulcer*) or (crural ulcer*) or (ulcus cruris) or (ulcer* cruris)) )
S1 (MH "Leg Ulcer+")
US National Institutes of Health Ongoing Trials Register (ClinicalTrials.gov)
compression OR stockings OR tights OR hosiery OR sock OR socks OR bandage OR wrap OR wrapping | Venous Leg Ulcer
compression OR stockings OR tights OR hosiery OR sock OR socks OR bandage OR wrap OR wrapping | Ulcer Venous
compression OR stockings OR tights OR hosiery OR sock OR socks OR bandage OR wrap OR wrapping | Varicose Ulcer
compression OR stockings OR tights OR hosiery OR sock OR socks OR bandage OR wrap OR wrapping | Venous Stasis Ulcer
compression OR stockings OR tights OR hosiery OR sock OR socks OR bandage OR wrap OR wrapping | Venous Ulceration
World Health Organization International Clinical Trials Registry Platform
compression OR stockings OR tights OR hosiery OR sock OR socks OR bandage OR wrap OR wrapping [Intervention] | Venous Leg Ulcer [Title]
compression OR stockings OR tights OR hosiery OR sock OR socks OR bandage OR wrap OR wrapping | Venous Leg Ulcer [Condition]
compression OR stockings OR tights OR hosiery OR sock OR socks OR bandage OR wrap OR wrapping | Ulcer Venous [Title]
compression OR stockings OR tights OR hosiery OR sock OR socks OR bandage OR wrap OR wrapping | Ulcer Venous [Condition]
compression OR stockins OR tights OR hosiery OR sock OR socks OR bandage OR wrap OR wrapping | Varicose Ulcer [Title]
compression OR stockings OR tights OR hosiery OR sock OR socks OR bandage OR wrap OR wrapping | Varicose Ulcer [Condition]
compression OR stockings OR tights OR hosiery OR sock OR socks OR bandage OR wrap OR wrapping | Venous Stasis Ulcer [Title]
compression OR stockings OR tights OR hosiery OR sock OR socks OR bandage OR wrap OR wrapping | Venous Stasis Ulcer [Condition]
compression OR stockings OR tights OR hosiery OR sock OR socks OR bandage OR wrap OR wrapping | Venous Ulceration [Title]
compression OR stockings OR tights OR hosiery OR sock OR socks OR bandage OR wrap OR wrapping | Venous Ulceration [Condition]
Appendix 2. Risk of bias
1 Risk‐of‐bias assessment (individually randomised controlled trials)
1. Was the allocation sequence randomly generated?
Low risk of bias
The investigators describe a random component in the sequence generation process such as: referring to a random number table; using a computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots.
High risk of bias
The investigators describe a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, for example: sequence generated by odd or even date of birth; sequence generated by some rule based on date (or day) of admission; sequence generated by some rule based on hospital or clinic record number.
Unclear
Insufficient information about the sequence generation process to permit judgement of low or high risk of bias.
2. Was the treatment allocation adequately concealed?
Low risk of bias
Participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based and pharmacy‐controlled randomisation); sequentially‐numbered drug containers of identical appearance; sequentially‐numbered, opaque, sealed envelopes.
High risk of bias
Participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on: using an open random‐allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure.
Unclear
Insufficient information to permit judgement of low or high risk of bias. This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definite judgement, for example if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque and sealed.
3. Blinding ‐ was knowledge of the allocated interventions adequately prevented during the study?
Low risk of bias
Any one of the following.
No blinding, but the review authors judge that the outcome and the outcome measurement are not likely to be influenced by lack of blinding.
Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken.
Either participants or some key study personnel were not blinded, but outcome assessment was blinded and the non‐blinding of others unlikely to introduce bias.
High risk of bias
Any one of the following.
No blinding or incomplete blinding, and the outcome or outcome measurement is likely to be influenced by lack of blinding.
Blinding of key study participants and personnel attempted, but likely that the blinding could have been broken.
Either participants or some key study personnel were not blinded, and the non‐blinding of others likely to introduce bias.
Unclear
Any one of the following.
Insufficient information to permit judgement of low or high risk of bias.
The study did not address this outcome.
4. Were incomplete outcome data adequately addressed?
Low risk of bias
Any one of the following.
No missing outcome data.
Reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias).
Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups.
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically‐relevant impact on the intervention effect estimate.
For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically‐relevant impact on observed effect size.
Missing data have been imputed using appropriate methods.
High risk of bias
Any one of the following.
Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups.
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically‐relevant bias in intervention effect estimate.
For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes enough to induce clinically‐relevant bias in observed effect size.
‘As‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation.
Potentially inappropriate application of simple imputation.
Unclear
Any one of the following.
Insufficient reporting of attrition/exclusions to permit judgement of low or high risk of bias (e.g. number randomised not stated, no reasons for missing data provided).
The study did not address this outcome.
5. Are reports of the study free of suggestion of selective outcome reporting?
Low risk of bias
Any of the following.
The study protocol is available and all of the study’s prespecified (primary and secondary) outcomes that are of interest in the review have been reported in the prespecified way.
The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were prespecified (convincing text of this nature may be uncommon).
High risk of bias
Any one of the following.
Not all of the study’s prespecified primary outcomes have been reported.
One or more primary outcomes are reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not prespecified.
One or more reported primary outcomes were not prespecified (unless clear justification for their reporting is provided, such as an unexpected adverse effect).
One or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis.
The study report fails to include results for a key outcome that would be expected to have been reported for such a study.
Unclear
Insufficient information to permit judgement of low or high risk of bias. It is likely that most studies will fall into this category.
6. Other sources of potential bias
Low risk of bias
The study appears to be free of other sources of bias.
High risk of bias
There is at least one important risk of bias. For example, the study:
had a potential source of bias related to the specific study design used; or
has been claimed to have been fraudulent; or
had some other problem.
Unclear
There may be a risk of bias, but there is either:
insufficient information to assess whether an important risk of bias exists; or
insufficient rationale or evidence that an identified problem will introduce bias
2 Risk‐of‐bias assessment (cluster‐randomised controlled trials)
1. Recruitment bias
Recruitment bias (or identification bias) is the bias that occurs in cluster‐RCTs if the personnel recruiting participants know individuals’ allocation, even when the allocation of clusters has been concealed appropriately. The knowledge of the allocation of clusters may lead to bias because the individuals' recruitment in cluster trials is often behind the clusters' allocation to different interventions; and the knowledge of allocation can determine whether individuals are recruited selectively.
This bias can be judged through considering the following questions.
Were all the individual participants identified/recruited before randomisation of clusters?
Is it likely that selection of participants was affected by knowledge of the intervention?
Were there baseline imbalances that suggest differential identification or recruitment of individual participants between arms?
2. Baseline imbalance
Baseline imbalance between intervention groups can occur due to chance, problems with randomisation, or identification/recruitment bias. The issue of recruitment bias has been considered above.
In terms of study design, the risk of chance baseline imbalance can be reduced by the use of stratified or pair‐matched randomisation. Minimisation — an equivalent technique to randomisation — can be used to achieve better balance in cluster characteristics between intervention groups if there is a small number of clusters.
Concern about the influence of baseline imbalance can be reduced if trials report the baseline comparability of clusters, or statistical adjustment for baseline characteristics.
3. Loss of clusters
Similar to missing outcome data in individually‐randomised trials, bias can occur if clusters are completely lost from a cluster trial, and are omitted from the analysis.
The amount of missing data, the reasons for missingness and the way of analysing data given the missingness should be considered in assessing the possibility of bias.
4. Incorrect analysis
Data analyses which do not take the clustering into account, in cluster trials will be incorrect. Such analyses lead to a 'unit of analysis error' and over‐precise results (too small standard error) and too small P values. Although these analyses will not result in biased estimates of effect, if not correctly adjusted they will lead to too much weight allocated to cluster trials in a meta‐analysis.
Note that the issue of analysis may not lead to concern any more and will not be considered substantial if approximate methods are used by review authors to address clustering in data analysis.
5. Comparability with individually‐randomised trials
In the case that a meta‐analysis includes, for example, both cluster‐ and individually‐randomised trials, potential differences in the intervention effects between different trial designs should be considered. This is because the 'contamination' of intervention effects may occur in cluster‐randomised trials, which would lead to underestimates of effect. The contamination could be known as a 'herd effect', i.e. within clusters, individuals' compliance with using an intervention may be enhanced, which in turn affects the estimation of effect.
Appendix 3. Data for complete wound healing outcomes
| Study | Comparison | Time‐to‐complete wound healing | Proportion of wounds completely healed | Comments |
| Charles 1991 | Group 1. Compression (short‐stretch bandage) Group 2. No compression |
‐ | Leg ulcers completely healed Group 1. 71% Group 2. 25% |
Unit of analysis is probably ulcers. The authors concluded "the leg ulcers treated with the short‐stretch compression bandage had a statistically significant (chi‐square test) higher healing rate than those treated in the control group." |
| Daróczy 2006 | Group 1. Compression (topical povidone‐iodine plus compression) Group 2. No compression (topical povidone‐iodine without compression) |
‐ | Group 1. 82% of 21 patients Group 2. 62% of 21 patients |
|
| Kikta 1988 | Group 1. Compression (Unna’s boot) Group 2. No compression (hydroactive dressing) |
Estimated results using Tierney 2007 methods: HR 2.38 95% CI 1.23 to 4.60 (lnHR 0.87 and selnHR 0.34) |
Data included in Analysis 1.2 Group 1. 33/42 ulcers healed Group 2. 21/45 ulcers healed Data included in the associated sensitivity analysis of assuming missing data had unhealed leg ulcers Group 1. 21/42 ulcers healed Group 2. 15/45 ulcers healed. |
Regarding the proportion of wounds completely healed, the difference in the data is due to the different assumptions applied for data analysis (see Sensitivity analysis). |
| Morrell 1998 | Group 1. Compression (4‐layer bandaging) Group 2. No compression (usual care) |
Group 1 compared with Group 2. Log rank test statistic 4.90 (df =1, P = 0.03); Univariate Cox analysis: HR 1.45 (95% CI 1.04 to 2.03); Multivariate Cox analysis: HR 1.65 (95% CI 1.15 to 2.35). Median healing time: Group 1. 20 weeks Group 2. 43 weeks Mean number of weeks the participants were free from ulcers: Group 1. 20.1 weeks Group 2. 14.2 (difference 5.9; 95% CI 1.2 to 10.5) |
Number (%) patients with complete healing at 12 months Group 1. 78/120 (65%) Group 2. 62/113 (55%) |
|
| O'Brien 2003 | Group 1. Compression (4‐layer bandage) Group 2. No compression |
Group 1 compared with Group 2 HR 1.8 (95% CI 1.2 to 2.9) |
Kaplan–Meier estimate of the proportion healed at 3 months Group 1. 54% Group 2. 34% (P < 0·001). |
|
| Rubin 1990 | Group 1. Compression (Unna’s boot) Group 2. No compression (polyurethane foam dressing) |
‐ | ITT analysis: Group 1. 18/19 (94.7%) patients with ulcers healed Group 2. 7/17 (41.2%). Complete cases: Group 1. 18/19 (94.7%) Group 2. 7/8 (87.5%) (Chi‐squared = 8.2, P < 0.005). |
|
| Taradaj 2007 | Group 1. Compression Group 2. No compression |
‐ | Narratives: No statistical difference in the pre‐ and post‐treatment changes of total surface area, volume, length, width, field, and granulation surface area among groups (P‐values of all between‐group comparisons > 0.05); the percentage of weekly wound surface change rates; and the percentage of weekly ulcer volume change rates. |
|
| Taylor 1998 | Group 1. Compression Group 2. |
Results calculated using the methods in Tierney 2007: HR 4.54 (95% CI 1.65 to 12.49); lnHR 1.51 and selnHR 0.52. Median healing time Group 1. 55 days Group 2. 84 days. (Lee‐Desu statistic 8.603, P = 0.0034). |
Data used in Analysis 1.2 Group 1. 14/18 Group 2. 7/18 Data used in the associated sensitivity analysis of assuming missing data had unhealed leg ulcers Group 1. 12/18 Group 2. 3/18 |
Regarding the proportion of wounds completely healed, the difference in the data is due to the different assumptions applied for data analysis (see Sensitivity analysis). |
| Wong 2008a | Group 1. Compression (short‐stretch bandage, and 4‐layer bandage) Group 2. No compression |
‐ | Data used in Analysis 1.2 Group 1. 15/20 Group 2. 5/10 Data used in the associated sensitivity analysis of assuming missing data had unhealed leg ulcers Group 1. 13/20 Group 2. 3/10. |
Regarding the proportion of wounds completely healed, the difference in the data is due to the different assumptions applied for data analysis (see Sensitivity analysis). |
| Wong 2008b | Group 1. Compression (short‐stretch bandage) Group 2. Compression (4‐layer bandage) Group 3. No compression |
Cox regression adjusted for age, initial ulcer size, and ulcer duration (hazard ratios for healing for Group 1 and Group 2 relative to Group 3) HR 2.72 (95% CI= 1.53‐4.86); 3.14 (95% CI= 1.74‐5.67) Survival time Mean 7.831 weeks (SE 0.489) for SSB, 8.557 (0.430) for 4LB, 10.378 (0.383) for control. |
Data analysed in Analysis 1.2: Group 1. 48/60 Group 2. 50/60 Group 3. 23/60. Data used in the associated sensitivity analysis of assuming missing data had unhealed leg ulcers Group 1. 38/60 Group 2. 36/60 Group 3. 17/60. |
Regarding the proportion of wounds completely healed, the difference in the data is due to the different assumptions applied for data analysis (see Sensitivity analysis). |
| Wong 2012 | Group 1. Compression (short‐stretch bandage) Group 2. Compression (4‐layer bandage) Group 3. No compression |
Time‐to‐complete wound healing Group 1. mean 9.8 (SD 0.77) weeks Group 2. 10.4 (0.80) Group 3. 18.3 (0.86) |
Week 12: Group 1. 66.4% (71 ⁄ 107) Group 2. 59.8% (64 ⁄ 107) Group 3. 28.0% (30 ⁄ 107). Week 24: Group 1. 72.0% (77 ⁄ 107) Group 2. 67.3% (72 ⁄ 107) Group 3. 29.0% (31 ⁄ 107) |
Appendix 4. Sensitivity analyses
| Sensitivity analysis | Studies | Participants | Effect estimate |
| Outcome: Time‐to‐complete wound healing | |||
|
4 | 553 |
|
|
5 | 733 |
|
|
4 | 649 |
|
| Outcome: Proportion of wounds completely healed | |||
|
10 | 1215 |
|
|
8 | 1005 |
|
|
10 | 1215 |
|
|
9 | 1131 |
|
Data and analyses
Comparison 1. Compression bandages or stockings compared with no compression.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Time‐to‐complete wound healing | 5 | Hazard Ratio (IV, Random, 95% CI) | 2.17 [1.52, 3.10] | |
| 1.2 Proportion of wounds completely healed during follow‐up | 8 | 1123 | Risk Ratio (M‐H, Random, 95% CI) | 1.77 [1.41, 2.21] |
| 1.3 Adverse events | 3 | 588 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.25, 3.80] |
| 1.4 Participant health‐related quality of life/health status | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.4.1 SF‐12 (physical component) | 2 | 426 | Mean Difference (IV, Random, 95% CI) | 2.46 [‐1.62, 6.54] |
| 1.4.2 SF‐12 (mental component) | 2 | 426 | Mean Difference (IV, Random, 95% CI) | ‐0.74 [‐2.57, 1.09] |
| 1.4.3 SF‐12 (functional status) | 1 | 276 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐2.55, 1.35] |
| 1.4.4 Charing Cross Venous Ulcer Questionnaire (total score) | 2 | 426 | Mean Difference (IV, Random, 95% CI) | ‐6.87 [‐13.10, ‐0.64] |
| 1.5 Mean pain score | 3 | 495 | Mean Difference (IV, Random, 95% CI) | ‐1.39 [‐1.79, ‐0.98] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Cardoso 2019.
| Study characteristics | ||
| Methods |
Study objective: to evaluate the oedema evolution of the venous ulcer–affected lower limb by means of electric bioimpedance with the use of the Unna’s boot and the non‐compressive dressing Trial design (e.g. parallel group) including research sites: cross‐over, single site Follow‐up period: 1 day Number of arms: 2 Study start date and end date: September 2014 to December 2016 Care setting: not described |
|
| Participants |
Study population: adults with venous leg ulcers Eligibilitycriteria: people aged 18 years or older with leg ulcers and a history and physical examination compatible with chronic venous disease included. Exclusion criteria: a history suggestive of chronic arterial disease, active infections, and joint immobility excluded. Sex (M:F): 0:11 Age (years): 50 to 76 years (mean age: 63 years; SD = 7.5 years). Duration of leg ulcers: not described Baseline leg ulcer area: not described Group difference: not described Total number of participants: 15 legs of 11 individuals Unit of analysis (including number of ulcers per person): ulcers/legs Unit of randomisation (e.g. leg ulcer, limb, or participant): legs |
|
| Interventions |
Intervention characteristics Unna’s boot
Conventional dressing
|
|
| Outcomes |
Time‐to‐complete wound healing
Proportion of wounds completely healed during follow‐up
Adverse events
Participant health‐related quality of life/health status
Cost effectiveness
Mean pain score
Outcomes that are not considered in this review but reported in trials:
|
|
| Identification |
Publication type/ status (e.g. conference abstract): full paper Trial protocol: not described Source of funding: not described Country of origin: Brazil Comments: this paper formed part of the thesis ‘‘Unna boot therapy in reducing edema in patients with venous injuries’’, Medicine School of Sao Jose do Rio Preto, 2018. The author was contacted to request outcome data but no data on relevant outcomes were provided, although the authors replied that ulcer healing outcome was observed Contact information: Luciana Ventura Cardoso, Av. Constituicao, 1306, Boa Vista, Sao Jose do Rio Preto, SP CEP 15025‐120, Brazil. Tel.: +55 17 991233647. (E‐mail: lu_famerp@hotmail.com). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “The order of events considering the use of Unna’s boot and traditional dressing was randomly assigned by chance and drawing a number from an envelope determined the type of dressing to be used until the next evaluation.” Comment: unclear risk of bias because the method of generating random numbers is not clear |
| Allocation concealment (selection bias) | Unclear risk | Quote: “The order of events considering the use of Unna’s boot and traditional dressing was randomly assigned by chance and drawing a number from an envelope determined the type of dressing to be used until the next evaluation.” Comment: unclear risk of bias because it is unclear how concealment is implemented |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: no information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no information provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no information provided |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were prespecified |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias |
Charles 1991.
| Study characteristics | ||
| Methods |
Study objective: to compare the results of the short‐stretch bandage application with the present treatment being used for venous leg ulcers in the locality Trial design (e.g. parallel group) including research sites: parallel, community locality of Paddington and North Kensington, UK Follow‐up period: 3 months Number of arms: 2 Study start date and end date: not described Care setting: community |
|
| Participants |
Study population: community patients with an APBI of greater than 0.8, measured by vascular flow detector. Eligibilitycriteria: venous leg ulcer patients in the community locality of Paddington and North Kensington with an APBI of greater than 0.8, measured by vascular flow detector Sex (M:F): not described Age (years): mean 78 (range 55 to 99) in short‐stretch bandage and 75 (range 37 to 91) in control Duration of leg ulcers: on average 32 months (range 4 months to 28 years) in short‐stretch bandage and 25 (range 4 months to 10 years) in control Baseline leg ulcer area: mean 12 cm2 (range 1.5 to 52) in short‐stretch bandage and 15 (range 1 to 88) in control Group difference: not described Total number of participants: 53 patients (no. of ulcers unspecified) Unit of analysis (including number of ulcers per person): probably ulcer Unit of randomisation (e.g. leg ulcer, limb, or participant): patients |
|
| Interventions |
Intervention characteristics Short‐stretch bandage
Control
|
|
| Outcomes |
Time‐to‐complete wound healing
Proportion of wounds completely healed during follow‐up
Adverse events
Participant health‐related quality of life/health status
Cost effectiveness
Mean pain score
Outcomes that are not considered in this review but reported in trials:
|
|
| Identification |
Publication type/ status (e.g. conference abstract): full paper (Charles 1991); short report (Charles 1992 (see Charles 1991)) Trial protocol: not provided Source of funding: not described; Lohmann UK, Stone, Aylesbury supplying the bandages, padding and the use of an Oxford monitor for this project Country of origin: UK Comments: the authors stated this paper was part of a research project submitted to the Parkside Health Authority (the project report was not obtained) Contact information: Hildegard Charles, BSc NDN PWT, Paddington and North Kensington locality |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Patients with an ankle pressure index < 0.8 were randomly divided into a control and an experimental group" Comment: unclear risk of bias because of the lack of information on random number generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: no information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no information provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no information provided |
| Selective reporting (reporting bias) | Unclear risk | Comment: no information provided |
| Other bias | High risk | Comment: high risk of bias because the unit of randomisation was participants but the unit of analysis was probably leg ulcers |
Daróczy 2006.
| Study characteristics | ||
| Methods |
Study objective: to assess the effectiveness of (1) topical povidone‐iodine with and (2) without compression bandages, (3) to compare the efficacy of systemic antibiotics and topical antimicrobial agents to prevent the progression of superficial skin ulcers Trial design (e.g. parallel group) including research sites: prospective, randomised controlled study Follow‐up period: 12 weeks Number of arms: 3 (only 2 included in this review as 3rd group assessed antibiotic use) Study start date and end date: November 2003 to November 2004 Care setting: not reported |
|
| Participants |
Study population: ulcerated stasis dermatitis due to chronic venous insufficiency. Their clinical stage was determined by clinical, aetiological, anatomical and pathological classification Eligibilitycriteria: ulcerated stasis dermatitis due to chronic venous insufficiency Sex (M:F): not reported Age (years): 58 ± 8 years Duration of leg ulcers: not reported Baseline leg ulcer area: sizes of the superficial ulcers < 5 cm Group difference: not reported Total number of participants: 42 in relevant arms (a further 21 participants were included in an antibiotic arm) Unit of analysis (including number of ulcers per person): participants Unit of randomisation (e.g. leg ulcer, limb, or participant): participants |
|
| Interventions |
Intervention characteristics Local povidone‐iodine (Betadine) with compression
Local povidone‐iodine (Betadine) without compression
|
|
| Outcomes |
Time‐to‐complete wound healing
Proportion of wounds completely healed during follow‐up
Adverse events
Participant health‐related quality of life/health status
Cost effectiveness
Mean pain score
Outcomes that are not considered in this review but reported in trials:
|
|
| Identification |
Publication type/ status (e.g. conference abstract): full paper Trial protocol: not reported Source of funding: not described Country of origin: Hungary Contact information: Prof. Dr. Judit Daroczy, Department of Dermatology and Lymphology, St. Stephan Hospital, Jahn Ferenc u. 62‐66. HR‐1195, Budapest. +36 1280 13 68 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “… patients were enrolled in this prospective randomised controlled study ...” Comment: unclear risk of bias because random sequence generation not specified |
| Allocation concealment (selection bias) | Unclear risk | Quote: “… patients were enrolled in this prospective randomised controlled study ...” Comment: unclear risk of bias because allocation concealment not reported/specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: no information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome group: healing rate of superficial, infected ulcers Comment: all participants were included in the analysis |
| Selective reporting (reporting bias) | High risk | Comment: the authors claimed to evaluate time to ulcer healing but did not present relevant data |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias |
Eriksson 1984a.
| Study characteristics | ||
| Methods |
Study objective: to evaluate different methods of topical treatment of venous leg ulcers Trial design (e.g. parallel group) including research sites: parallel group Follow‐up period: 8 weeks Number of arms: 3 Study start date and end date: not given Care setting: not reported |
|
| Participants |
Study population: people with venous leg ulcers. Eligibilitycriteria: exclusion criteria are overt diabetes mellitus, manifest arterial insufficiency, clinical picture of erysipelas or cellulitis Sex (M:F): overall 13:40 (among all 53 participants who were enrolled in an unrelated trial prior to the compression trial) Age (years): mean 70.1 (among all 53 participants who were enrolled in an unrelated trial prior to the compression trial) Duration of leg ulcers: not reported Baseline leg ulcer area: not reported Group difference: comparable in terms of all variables except for high blood glucose levels, and history of previous thrombosis (among participants of the trial 1) Total number of participants: 44 participants in the compression trial Unit of analysis (including number of ulcers per person): participants Unit of randomisation (e.g. leg ulcer, limb, or participant): participants |
|
| Interventions |
Intervention characteristics Porcine skin dressing
Metallina aluminium foil dressing
Double‐layer bandage
|
|
| Outcomes |
Time‐to‐complete wound healing
Proportion of wounds completely healed during follow‐up
Adverse events
Participant health‐related quality of life/health status
Cost effectiveness
Mean pain score
Outcomes that are not considered in this review but reported in trials:
|
|
| Identification |
Publication type/ status (e.g. conference abstract): full paper Trial protocol: not reported Source of funding: not described Country of origin: Sweden Contact information: G. Eriksson, Departments of Dermatology, Dandeyd Hospital, Stockholm, Sweden |
|
| Notes | Eriksson 1984a presented results of 2 trials: the 2‐week trial 1 compared 0.9% sodium chloride in sterile water with dextranomer beads through randomising 53 participants with venous leg ulcers and 9 patients excluded because of ulcer healing or reasons unrelated to the trial 2 prior to the conduct of trial 2. With the remaining 44 participants, trial 2 was undertaken to compare 2 dressing methods with a compression bandage method. Only data from trial 2 were extracted for this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "... the patients were randomised for three different treatments." Comment: unclear risk of bias because the randomisation method is not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Outcome group: ulcer healing Quote: "The investigation was designed as a randomised open trial" Comment: high risk of bias because this trial is claimed to be "open" label. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome group: ulcer healing Quote: "Stereophotogrammetry was used as an objective method of measurement of the healing process" Comment: low risk of bias because ulcer outcomes are measured using the objective stereophotogrammetry as the authors claim |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no information provided. |
| Selective reporting (reporting bias) | Low risk | Comment: the authors claimed to evaluate time to ulcer healing but did not present relevant data. The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were prespecified |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias. |
Groenewald 1984.
| Study characteristics | ||
| Methods |
Study objective: to compare hydrocolloid dressings with conventional treatment (compression bandage) for the treatment of venous leg ulcers Trial design (e.g. parallel group) including research sites: parallel group Follow‐up period: 8 weeks Number of arms: 2 Study start date and end date: not given Care setting: outpatient setting |
|
| Participants |
Study population: people with venous stasis ulcers Eligibilitycriteria: not given Sex (M:F): overall ratio 1:3 among overall clinic population (not for eligible participants) Age (years): not described Duration of leg ulcers: some with recent onset; most with more than 6 months and some for many years Baseline leg ulcer area: not given Group difference: not given Total number of participants: 72 participants Unit of analysis (including number of ulcers per person): participants Unit of randomisation (e.g. leg ulcer, limb, or participant): participants |
|
| Interventions |
Intervention characteristics Hydrocolloid dressings
Conventional treatment (compression bandage)
|
|
| Outcomes |
Time‐to‐complete wound healing
Proportion of wounds completely healed during follow‐up
Adverse events
Participant health‐related quality of life/health status
Cost effectiveness
Mean pain score
Outcomes that are not considered in this review but reported in trials:
|
|
| Identification |
Publication type/ status (e.g. conference abstract): full paper Trial protocol: not reported Source of funding: not described Country of origin: South Africa Contact information: J.H. Groenewald, Departments of Vascular Surgery, University of Stellenbosch, South Africa |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were selected randomly for treatment either with hydrocolloid dressing or by conventional methods" Comment: unclear risk of bias because the randomisation method is not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Outcome group: ulcer healing Quote: "The nature of the dressing made a double‐blind study impractical" Comment: high risk of bias because the authors claimed it is challenging to blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome group: ulcer healing Quote: "... reduction in ulcer size as the main criterion for the evaluation of results. This could be measured fairly accurately by a technician who was not otherwise involved in the trial" Comment: low risk of bias because study authors took measures to reduce the risk of detection bias in measuring ulcer sizes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no information provided |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were prespecified |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias |
Kikta 1988.
| Study characteristics | ||
| Methods |
Study objective: not given Trial design (e.g. parallel group) including research sites: parallel, randomised controlled trial, multi‐site (University of Illinois Hospital, Westside Veterans Administration Hospital, and Cook County Hospital Vascular Surgery Clinics, USA) Follow‐up period: 6 months Number of arms: 2 Study start date and end date: not described (randomisation between February 1986 and January 1987) Care setting: hospital (clinics) |
|
| Participants |
Study population: leg ulcers caused by chronic venous insufficiency Eligibilitycriteria: included people with leg ulcers caused by chronic venous insufficiency. Excluded those with the presence of arterial insufficiency as measured by Doppler‐derived ABPI of less than 0.7, uncontrolled diabetes mellitus, the use of cancer chemotherapeutic agents or systemic steroids, recent venous surgery, infected ulcers, and inability to comply with treatment or follow‐up Sex (M:F): not described Age (years): not described Duration of leg ulcers: mean 45 (SEM 12) weeks in hydroactive dressing (number of ulcers unspecified (n = 39 ulcers)); 51 (17) in Unna’s boot (number of ulcers unspecified (n = 30 ulcers)), Student’s t test P‐value = 0.77 Baseline leg ulcer area: mean 8.6 (SEM 2.1) cm2 in hydroactive dressing (number of ulcers unspecified (n = 39 ulcers)); 9.0 (2.2) in Unna’s boot (number of ulcers unspecified (n = 30 ulcers)), Student’s t test P‐value = 0.88 Group difference: no statistically significant differences between groups in many variables (e.g. age; sex; race; type of previous ulcer treatment; pre‐randomisation use of antibiotics; origin of chronic venous insufficiency; the incidence of previous venous, arterial, or orthopaedic operations; prior use of elastic stockings; the incidence of ischaemic heart disease, congestive heart failure, diabetes mellitus, hypertension, pulmonary, renal, and hepatic diseases; the use of oral contraceptives or tobacco; the presence of obesity or alcoholism; elevated levels of serum haemoglobin, glucose, albumin, and creatinine; whether the ulcer was new or recurrent; ABIs; and PPG‐VRT) Total number of participants: 84 participants with 87 ulcers Unit of analysis (including number of ulcers per person): ulcers, approximately 1 ulcer per person Unit of randomisation (e.g. leg ulcer, limb, or participant): participants |
|
| Interventions |
Intervention characteristics Unna’s boot
Hydroactive dressing
|
|
| Outcomes |
Time‐to‐complete wound healing
Proportion of wounds completely healed during follow‐up
Adverse events
Participant health‐related quality of life/health status
Cost effectiveness
Mean pain score
Outcomes that are not considered in this review but reported in trials:
|
|
| Identification |
Publication type/ status (e.g. conference abstract): full paper Trial protocol: not described Source of funding: not described Country of origin: USA Comments: this study was presented at the combined breakfast program of the Society for Vascular Surgery and the International Society for Cardlovascular Surgery, North American Chapter, Toronto, Ontario, Canada, 09 June 1987. However, the abstract is not available Contact information: D. Preston Flanigan, M.D., Chief, Division of Vascular Surgery (m/c 957), University of Illinois at Chicago, 1740 West Taylor St., Suite 2200, Chicago, IL 60612 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “... patients with leg ulcers caused by chronic venous insufficiency were randomised to receive local wound care with either UB or HD ...” Comment: unclear risk of bias because the method of random sequence generation is not specified |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: no information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no information provided Outcomes: pain Comment: high risk of bias because this outcome is self‐rated by participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “Eighteen patients withdrew from study participation within 2 weeks of randomization, leaving 69 ulcers in 66 patients available for analysis” Quote: “There was no statistically significant difference (p = 0.11, FET) between the number withdrawing from the HD group (6 of 45) and the UB group (12 of 42) ... Despite the greater withdrawal rate from the UB group, there was still a statistically significant difference in healing rates (p = 0.01, log rank test) between ...” Outcome: proportion of wounds completely healed Comment: low risk of bias because despite a high proportion of dropouts, results are consistent between life‐table analysis that incorporates dropouts, and Chi2 test that excludes dropouts Outcome: pain Comment: high risk of bias because dropout rate is higher in Unna’s boot group than hydroactive dressing group and pain could be one of likely reasons why dropouts occur Outcome: other outcomes Comment: unclear risk of bias. |
| Selective reporting (reporting bias) | High risk | Comment: high risk of bias because participant compliance seems prespecified but its results are not presented |
| Other bias | High risk | Comment: high risk of bias because the unit of randomisation was participants but the unit of analysis was probably leg ulcers |
Morrell 1998.
| Study characteristics | ||
| Methods |
Study objective: to establish the relative cost effectiveness of community leg‐ulcer clinics that use 4‐layer compression bandaging versus usual care provided by district nurses Trial design (e.g. parallel group) including research sites: parallel, multi‐site (8 clinics of 4 community trusts in Trent) Follow‐up period: 12 months Number of arms: 2 Study start date and end date: recruitment from September 1994 to May 1995 Care setting: 8 community‐based research clinics in 4 trusts in Trent, UK |
|
| Participants |
Study population: people with venous leg ulcers Eligibilitycriteria: enrolled those with 1 or more venous ulcers on 1 or both lower limbs above the foot; their ulcers with at least 3 months duration, and assessed by the Doppler technique. Patients with an ABPI of 0.8 or less, indicating peripheral arterial disease, were excluded Sex (M:F): 43:77 in 4‐layer bandaging and 35:78 in usual care Age (years): mean 73.2 (SD 11.6) in usual care; 73.8 (10.9) in 4‐layer bandaging Duration of leg ulcers: mean 29.7 (SD 82.3) months in usual care; 27.5 (53.8) in 4‐layer bandaging Baseline leg ulcer area: mean 16.9 (SD 40.8) cm2 in usual care; 16.2 (28.9) in 4‐layer bandaging Group difference: no difference Total number of participants: 233 Unit of analysis (including number of ulcers per person): participants Unit of randomisation (e.g. leg ulcer, limb, or participant): participants |
|
| Interventions |
Intervention characteristics 4‐layer bandaging in a leg‐ulcer clinic (Charing Cross)
Usual care at home by the district nursing service
|
|
| Outcomes |
Time‐to‐complete wound healing
Proportion of wounds completely healed during follow‐up
Adverse events
Participant health‐related quality of life/health status
Cost effectiveness
Mean pain score
Outcomes that are not considered in this review but reported in trials:
|
|
| Identification |
Publication type/ status (e.g. conference abstract): full papers (Brereton 1997; Collins 1997; Collins 1998; Morrell 1996; Morrell 1998) Trial protocol: not provided Source of funding: funded by the former Trent Regional Health Authority (now NHS Executive Trent) Country of origin: UK Comments: this study has 5 publications and all sources have consistent results Contact information: Dr Morrell j.morrell1@sheffield.ac.uk |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “A random assignment schedule and serially numbered, sealed, opaque allocation envelopes were prepared in advance for each of the eight clinic sites” Comment: unclear risk of bias because the random‐number generation method is not described |
| Allocation concealment (selection bias) | Low risk | Quote: “A random assignment schedule and serially numbered, sealed, opaque allocation envelopes were prepared in advance for each of the eight clinic sites” Comment: low risk of bias because allocation is likely concealed properly |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Outcome group: all outcomes Comment: no information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome group: all outcomes Comment: no information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome group: all outcomes Quote: “All the data analysis was by intention to treat” Comment: low risk of bias because ITT analysis was performed |
| Selective reporting (reporting bias) | Unclear risk | Comment: unclear risk of bias because of the inconsistency in terms of primary outcome measurement between methods and results (the measure of “time to complete healing of all ulcers” claimed in methods, but “time to initial leg ulcer healing” presented in results) |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias |
O'Brien 2003.
| Study characteristics | ||
| Methods |
Study objective: to compare the cost effectiveness of 4‐layer bandaging with that of alternative dressings available for venous leg ulcers in a pragmatic randomised clinical trial Trial design (e.g. parallel group) including research sites: parallel Follow‐up period: 12 weeks Number of arms: 2 Study start date and end date: recruitment from April 1999 to August 2000 Care setting: community |
|
| Participants |
Study population: people with a venous leg ulcer Eligibilitycriteria: those with a venous leg ulcer who were not being treated with 4‐layer bandaging; venous ulcers defined with clinical evidence of venous disease, the resting ABPI = 0.9 or greater, and no other cause identified Sex (M:F): 35:65 in 4LB; 33:67 in control Age (years): mean 71.7 (SD 9.8) in 4LB; 71.4 (11.5) in control Duration of leg ulcers: median 9 (IQR 4 to 27) weeks in 4LB; 11 (5 to 28) in control Baseline leg ulcer area: median 3.5 (IQR 1.3 to 8.1) cm2 in 4LB; 2.7 (1.6 to 6.2) in control Group difference: no difference Total number of participants: 200 Unit of analysis (including number of ulcers per person): participant Unit of randomisation (e.g. leg ulcer, limb, or participant): participant |
|
| Interventions |
Intervention characteristics Four‐layer bandaging
Usual care (alternative dressings)
|
|
| Outcomes |
Time‐to‐complete wound healing
Proportion of wounds completely healed during follow‐up
Adverse events
Participant health‐related quality of life/health status
Cost effectiveness
Mean pain score
Outcomes that are not considered in this review but reported in trials:
|
|
| Identification |
Publication type/ status (e.g. conference abstract): full papers (see O'Brien 2003) Trial protocol: not available Source of funding: Smith & Nephew Ltd provided an educational grant to fund this study Country of origin: Ireland Comments: the authors presented the cost per leg ulcer healed for economic analysis in which the benefit was measured as leg ulcer healed Contact information: Mr P. E. Burke, Department of Vascular Surgery, St John’s Hospital, Limerick, Ireland (e‐mail: vsherlock@mwhb.ie). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “… a random ‘intervention’ or ‘control’ list was generated for 200 patients by computer …” Comment: low risk of bias because of the use of a proper randomisation method |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Before the study began, a random ‘intervention’ or ‘control’ list was generated for 200 patients by computer, and the results were entered sequentially into sealed numbered envelopes. These envelopes were assigned to consecutive patients once consent had been obtained” Comment: unclear risk of bias because they do not describe if envelopes are opaque |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: no information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome group: ulcer healing Quote: “The ulcerated area was measured and photographed by the research officer” Quote: “a photograph of the site was taken to provide an objective review of outcome” Comment: low risk of bias because attempts are made to reduce the risk of detection bias Outcome group: cost Comment: no information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome group: ulcer healing and cost analysis Quote: “Intention‐to‐treat analysis was carried out” Quote: “Data missing for two patients” in Table 3 of O'Brien 2003 (for cost). Comment: low risk of bias because of ITT analysis performed and very low rate of missing data (2 of 100 in 4LB) for cost analysis Outcome group: quality of life Comment: unclear risk of bias because the rates of missing data are 15% in 4LB and 5% in control. The missing is due to ulcer healing among them and questionnaires only completed for those with unhealed ulcers at 6 weeks (92.9%, 79/85 in 4LB; and 95.8%, 91/95 in control) |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were prespecified |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias |
Rubin 1990.
| Study characteristics | ||
| Methods |
Study objective: not given Trial design (e.g. parallel group) including research sites: multi‐site, randomised, prospective, blinded trial. Cleveland Ohio Veterans Administration Medical Centre, University Hospitals of Cleveland, and Cleveland Metropolitan General Hospital, USA Follow‐up period: 12 months Number of arms: 2 Study start date and end date: not described Care setting: hospital |
|
| Participants |
Study population: ambulatory patients with lower‐extremity chronic venous stasis ulceration Eligibilitycriteria: patients with lower‐extremity chronic venous stasis ulceration enrolled; those with history of non‐compliance, presence of significant lower‐extremity arterial insufficiency (as determined by Doppler ABPIs of less than 0.8), history of collagen vascular disease/uncontrolled diabetes/other ongoing dermatological disorders/chronic corticosteroid therapy excluded Sex (M:F): not described Age (years): not described Duration of leg ulcers: not described Baseline leg ulcer area: ranged from 6.0 cm ‐ 270 cm2 (mean 32.2cm2) for Polyurethane Foam Dressing (PFD) group. Ranged from 0.02 – 600 cm2 (mean 76.0 cm2) for Unna Boot group. Students t test P = 0.03 Group difference: ulcers of Unna boot group larger than those of dressing group. Initial bacterial culture results were positive in 13 (76.4%) of 17 limbs from PFD group and 12 (63.1%) of 19 limbs from Unna boot group – not statistically significant Total number of participants: 36 participants Unit of analysis (including number of ulcers per person): participants, and wound healing rates Unit of randomisation (e.g. leg ulcer, limb, or participant): participant |
|
| Interventions |
Intervention characteristics Polyurethane Foam Dressing
Unna’s boot
|
|
| Outcomes |
Time‐to‐complete wound healing
Proportion of wounds completely healed during follow‐up
Adverse events
Participant health‐related quality of life/health status
Cost effectiveness
Mean pain score
Outcomes that are not considered in this review but reported in trials:
|
|
| Identification |
Publication type/ status (e.g. conference abstract): full paper Trial protocol: not described Source of funding: not described Country of origin: USA Contact information: Dr Rubin, Department of Surgery, University Hospitals of Cleveland, 2074 Abington Road, Cleveland, OH 44106 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Each patient was randomised by the study coordinator to either a PFD or Unna boot dressing treatment protocol.” Comment: unclear risk of bias because the method of random‐sequence generation is not specified |
| Allocation concealment (selection bias) | Unclear risk | Quote: “The study coordinator did not see the randomization card and was therefore blinded as to the treatment cohort” Comment: unclear risk of bias because it is unclear if allocation is concealed so that investigators cannot foresee assignment of participants |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: no information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no information provided on who/how outcomes were assessed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome: complete wound healing Quote: “9 (52.9%) of 17 participants of group 1 withdrew from the study due to wound odor” Comment: low risk of bias because although 9/17 participants withdrew before study end from group 1 but no withdrawals from group 2, the authors performed ITT analysis for this outcome |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were prespecified |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias |
Taradaj 2007.
| Study characteristics | ||
| Methods |
Study objective: to evaluate the effect of sonotherapy and compression therapy compared with pharmacological treatment on the healing of venous ulcers after surgery Trial design (e.g. parallel group) including research sites: parallel group and single site Follow‐up period: 8 weeks Number of arms: 2 (of 3 arms) eligible Study start date and end date: not given Care setting: general, vascular and transplantation surgery clinic of an independent public hospital in Katowice, Poland |
|
| Participants |
Study population: people with venous ulcers who had had surgery using modified Babcock's method Eligibilitycriteria: Doppler blood‐flow testing of lower limb arteries undergone. Patients with ABPI higher than 0.9 included and those with ulcers of a different aetiology than the venous one excluded. Those with diabetes, atherosclerosis, rheumatoid arthritis, taking glycolyl steroids, and with metal implants in the ultrasound site excluded Sex (M:F): 9:16 compression; 13:11 control Age (years): 61.6 (8.3) and range 43 to 78 compression; 62.3 (9.5) and range 40 to 79 control Duration of leg ulcers: 36 (39) and range 6 to 176 compression; 32 (35) and range 2 to 120 control Baseline leg ulcer area: 24.4 (12.9) compression; 22.0 (15.5) control Group difference: comparable for all variables assessed Total number of participants: 49 (of 73 patients eligible for inclusion in this review) Unit of analysis (including number of ulcers per person): not given, probably ulcers Unit of randomisation (e.g. leg ulcer, limb, or participant): participants |
|
| Interventions |
Intervention characteristics Elastic bandage compression and pharmacotherapy
Control (pharmacological treatment)
|
|
| Outcomes |
Time‐to‐complete wound healing
Proportion of wounds completely healed during follow‐up
Adverse events
Participant health‐related quality of life/health status
Cost effectiveness
Mean pain score
Outcomes that are not considered in this review but reported in trials:
|
|
| Identification |
Publication type/ status (e.g. conference abstract): full paper (Polish) Trial protocol: not reported Source of funding: not described Country of origin: Poland Comments: ultrasound therapy plus pharmacological treatments were used as a third group in this trial. Data for this group were not extracted for this review, given it was not eligible Contact information: Jakub Taradaj, Chair and Department of Medical Biophysics, Silesian Medical University in Katowice, Poland (email: jtaradaj@slam.katowice.pl) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: unclear risk of bias because randomisation method is not specified. From translator: "... random assignment ..." |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: no information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no information provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no information provided |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were prespecified |
| Other bias | High risk | Comment: high risk of bias because the unit of randomisation was participants but the unit of analysis was probably leg ulcers |
Taylor 1998.
| Study characteristics | ||
| Methods |
Study objective: to compare healing rates and associated treatment costs of 4‐layer high‐compression bandaging and conventional management in the treatment of venous leg ulcers Trial design (e.g. parallel group) including research sites: parallel group Follow‐up period: 12 weeks Number of arms: 2 Study start date and end date: not given Care setting: hospital‐based leg ulcer service and community |
|
| Participants |
Study population: people with venous stasis ulcers Eligibilitycriteria: consecutive patients presenting with venous ulcers and an ABPI > 0.8 Sex (M:F): overall 11:19 among 30 complete cases; 7:9 in compression bandage and 4:10 in conventional management Age (years): median 73 (range 28 to 85) in compression and 77 (60 to 84) in conventional Duration of leg ulcers: 7 participants with ulcers < 6 months and 9 with > 6 months in compression; and 9 with < 6 months and 5 with > 6 months in conventional. Baseline leg ulcer area: median 5.4 (range 0.4 to 74.8) cm2 total area of all ulcers in compression; 4.2 (0.6 to 76.0) in conventional Group difference: no difference in any variables assessed Total number of participants: 36 patients (30 compliers analysed) Unit of analysis (including number of ulcers per person): participants Unit of randomisation (e.g. leg ulcer, limb, or participant): participants |
|
| Interventions |
Intervention characteristics Four‐layer compression bandage regimen
Conventional management
|
|
| Outcomes |
Time‐to‐complete wound healing
Proportion of wounds completely healed during follow‐up
Adverse events
Participant health‐related quality of life/health status
Cost effectiveness
Mean pain score
Outcomes that are not considered in this review but reported in trials:
|
|
| Identification |
Publication type/ status (e.g. conference abstract): full paper (Taylor 1998), 2 conference abstracts Trial protocol: not reported Source of funding: financial support from Medi (UK) Ltd; 3M Health Care providing Coban bandages Country of origin: UK Contact information: Mrs Adrienne Taylor, Clinical Nurse Specialist, Salford Community Trust, The Willows Centre for Health Care, Lords Avenue, Weaste, Salford M5 2JR, UK |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "... patients were randomly allocated to each treatment group using the method of minimization of prognostic factors" Comment: low risk of bias because of the use of a proper method equivalent to randomisation |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: no information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome group: ulcer healing Quote: "Weekly, each patient had the perimeter of their ulcer traced on to acetate and the area measured using a computerized planimeter. The change in ulcer area gave a quantitative measure of healing rate" Comment: low risk of bias because attempts made to reduce the risk of detection bias in measuring ulcer healing Outcome group: cost outcome Quote: "the nurse completed a purpose‐designed treatment inventory, detailing all the medications, consumables, distance travelled, travelling time, nursing grade and time to treat the patient" Comment: no information provided |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: high risk of bias because the overall proportion of dropouts reach 20% (2 of 18 in compression and 4 of 18 in conventional withdrew) and ITT analysis not performed (1 death in each group and 1 participant with ulcer healing before treatment could have been considered in analysis) |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were prespecified |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias |
Wong 2008a.
| Study characteristics | ||
| Methods |
Study objective: to examine the feasibility of the sampling method and to test the measuring instruments, data collection procedure, and study intervention Trial design (e.g. parallel group) including research sites: parallel, pilot, randomised trial, single site Follow‐up period: 12 weeks Number of arms: 3 Study start date and end date: not given Care setting: community (1 general outpatient clinic) |
|
| Participants |
Study population: older people with venous ulcer living in the community Eligibilitycriteria: inclusion of either men or women aged 55 years or older having confirmed venous leg ulcers (with partial or full‐thickness skin loss) by clinical and vascular assessment (i.e. Doppler); without necrotic tissue; ability to understand and communicate in Cantonese; ABPI ≥ 0.8 indicating suitability for compression bandaging; have not previously received compression bandage. Exclusion of people with ulcers > 14 cm x 14 cm, with an ulcer duration of < 2 months; those with 2 or multiple leg ulcers, with known history of sensitivity to wound dressing or bandage used; with concurrent administration of drugs that may affect ulcer healing, such as corticosteroids or chemotherapeutics, ulcer accompanied with neoplasm skin or tissue infection, and trauma such as burn or surgical incision, diabetic patient receiving oral hypoglycaemic or insulin therapy because reduced pressure is required (from Wong 2008b) Sex (M:F): 20:6 overall Age (years): mean 68.9 (SD 9.4) overall Duration of leg ulcers: mean 22 (SD 31) months overall Baseline leg ulcer area: mean 8.24 (SD 8.7) cm2 overall Group difference: not given Total number of participants: 30 participants (26 completed the pilot study) Unit of analysis (including number of ulcers per person): participants, each with 1 ulcer Unit of randomisation (e.g. leg ulcer, limb, or participant): participants |
|
| Interventions |
Intervention characteristics Four‐layer bandage
Short‐stretch bandage
Usual care
|
|
| Outcomes |
Time‐to‐complete wound healing
Proportion of wounds completely healed during follow‐up
Adverse events
Participant health‐related quality of life/health status
Cost effectiveness
Mean pain score
Outcomes that are not considered in this review but reported in trials:
|
|
| Identification |
Publication type/ status (e.g. conference abstract): PhD thesis (Wong 2008b) Trial protocol: not described Source of funding: funded by the Special Grant for Conducting Research Aboard, the Chinese University of Hong Kong (2004) Country of origin: Hong Kong Comments: this was a pilot study of Wong 2008b; and both Wong 2008a and Wong 2008b were reported in a single doctoral thesis. Therefore, some data items of Participants and Interventions were extracted from Wong 2008b. Most domains of risk‐of‐bias table were judged based on Wong 2008b Contact information: Irene KY Wong |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: unclear risk of bias because information provided is insufficient for this domain’s judgement |
| Allocation concealment (selection bias) | Unclear risk | Comment: unclear risk of bias because information provided is insufficient for this domain’s judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Outcome group: all outcomes Quote: "participants and interviewers (data collectors) were not informed of the treatment allocation of the study participants for the duration of the study" (Wong 2008b). Quote: "the interveners who performed wound dressing and/or bandaging were not blinded to the treatment groups. Blinding the interveners is not achievable ..." (Wong 2008b). Comment: high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: unclear risk of bias because information provided is insufficient for this domain’s judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome group: all outcomes Quote: “The attrition rate was 7.1%” (Wong 2008a). Comment: low risk of bias because of a low attrition rate |
| Selective reporting (reporting bias) | High risk | Comment: high risk of bias because although this is a pilot study; the study protocol is not available; but it is clear that the study authors measured some outcomes and presented baseline data only rather than week 12 outcome data |
| Other bias | Unclear risk | Comment: unclear risk of bias because this is a pilot study report and no further information is available for other sources of bias judgement |
Wong 2008b.
| Study characteristics | ||
| Methods |
Study objective: to compare the effects in venous leg‐ulcer patients between short‐stretch compression (SSB), 4‐layer compression bandaging (4LB), and usual care with no compression (UC) Trial design (e.g. parallel group) including research sites: parallel, randomised controlled trial, multi‐site Follow‐up period: 6 and 12 weeks Number of arms: 3 Study start date and end date: recruited from August 2004 to March 2006 Care setting: community involving 6 general outpatient clinics located at the Kowloon East Cluster |
|
| Participants |
Study population: older people with venous ulcer living in the community Eligibilitycriteria: inclusion of either men or women aged 55 years or older having confirmed venous leg ulcers (with partial or full‐thickness skin loss) by clinical and vascular assessment (i.e. Doppler); without necrotic tissue; ability to understand and communicate in Cantonese; ABPI ≥ 0.8 indicating suitability for compression bandaging; have not previously received compression bandage. Exclusion of patients with ulcers > 14cm x 14cm, with an ulcer duration of < 2 months; those with 2 or multiple leg ulcers, with known history of sensitivity to wound dressing or bandage used; with concurrent administration of drugs that may affect ulcer healing, such as corticosteroids or chemotherapeutics, ulcer accompanied with neoplasm skin or tissue infection, and trauma such as burn or surgical incision, diabetic patient receiving oral hypoglycaemic or insulin therapy because reduced pressure is required Sex (M:F): 121:59 overall Age (years): mean 69.3 (SD 9.8) overall Duration of leg ulcers: mean 31.6 (SD 44.8) months overall Baseline leg ulcer area: mean 8.1 (SD 8.8) cm2 overall Group difference: no significant differences between groups in variables explored (e.g. age; sex; ulcer duration; ulcer size) Total number of participants: 180 participants Unit of analysis (including number of ulcers per person): participants, each with 1 ulcer Unit of randomisation (e.g. leg ulcer, limb, or participant): participants |
|
| Interventions |
Intervention characteristics Four‐layer bandage
Short‐stretch bandage
Usual care
|
|
| Outcomes |
Time‐to‐complete wound healing
Proportion of wounds completely healed during follow‐up
Adverse events
Participant health‐related quality of life/health status
Cost effectiveness
Mean pain score
Outcomes that are not considered in this review but reported in trials:
|
|
| Identification |
Publication type/ status (e.g. conference abstract): PhD thesis (Wong 2008b) Trial protocol: not described Source of funding: funded by the Special Grant for Conducting Research Aboard, the Chinese University of Hong Kong (2004) Country of origin: Hong Kong Comments: the trial has a pilot study Wong 2008a Contact information: Irene KY Wong |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Eligible patients were randomly assigned to either one of the experimental groups or the control group by a random list ... A randomisation list with three treatment blocks was generated by a computer program before the study ...” Comment: low risk of bias because of the use of a proper method of generating random sequence. |
| Allocation concealment (selection bias) | Unclear risk | Quote: “The participants were allowed to open the envelope for grouping allocation only after the collection of baseline data. The patients were not informed whether they were assigned to an intervention or control group until the completion of the study. The strength of this allocation concealment in random trials ...” Comment: unclear risk of bias because information provided is insufficient for this domain’s judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Outcome group: all outcomes Quote: "participants and interviewers (data collectors) were not informed of the treatment allocation of the study participants for the duration of the study" Quote: "the interveners who performed wound dressing and/or bandaging were not blinded to the treatment groups. Blinding the interveners is not achievable ..." Comment: high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome group: ulcer healing and pain outcomes Quote: "This was a double‐blind study, in which the interviewers who were responsible for the pre‐test and post test data collections were not given any information regarding the group to which the participant was assigned" Quote: "The data collector then received the electronic file periodically and performed the perimeter tracing and area calculation using the Wound Measurement System software. As a result, the data collector assessed the ulcer size without seeing the participant ..." Comment: low risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome group: time to healing Quote: “... only analyse the data of those participants who had received the treatment ... an intention to treat analysis was adopted during the process of survival analysis only” Comment: low risk of bias because of the use of ITT analysis. Outcome group: all other outcomes including healing incidence Comment: high risk of bias because despite a moderate rate of attrition (30/180, 16.7%) the excluded cases had larger ulcer sizes than those completed |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were prespecified |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias |
Wong 2012.
| Study characteristics | ||
| Methods |
Study objective: to compare quality of life (QOL) aspects in venous leg ulcer patients over 55 years of age, of short‐stretch compression (SSB), 4‐layer compression bandaging (4LB) and usual care (UC) (moist wound‐healing dressing, no compression) Trial design (e.g. parallel group) including research sites: parallel, randomised controlled trial, multi‐site Follow‐up period: 12 and 24 weeks Number of arms: 3 Study start date and end date: patients recruited from May 2007 to November 2008 Care setting: community (9 general outpatient clinics in the New Territories East Cluster, Kowloon East Cluster and the Kowloon Central Cluster) |
|
| Participants |
Study population: leg ulcers caused by chronic venous insufficiency Eligibilitycriteria: inclusion of either men or women aged 55 years or older having confirmed venous leg ulcers (with partial or full‐thickness skin loss) by clinical and vascular assessment (i.e. Doppler); without necrotic tissue; ability to understand and communicate in Cantonese; ABPI ≥ 0.8 indicating suitability for compression bandaging. Exclusion of people with ulcers of < 5 cm2 or > 118 cm2, with an ulcer duration of less than 4 weeks or longer than 1 year; those with 2 or multiple leg ulcers, either on 1 or both legs; those with an ABPI < 0.8 and those with concurrent administration of drugs that may affect ulcer healing, such as corticosteroids or chemotherapeutics Sex (M:F): 206:115 overall Age (years): mean 71.7 (SD 8.5) overall Duration of leg ulcers: mean 27.4 (SD 43.7) months overall Baseline leg ulcer area: mean 8.2 (SD 11.0) cm2 overall Group difference: no significant differences between groups in variables explored (e.g. age; sex; ulcer duration; ulcer size) Total number of participants: 321 patients, each with 1 ulcer Unit of analysis (including number of ulcers per person): participants, each with 1 ulcer Unit of randomisation (e.g. leg ulcer, limb, or participant): patients |
|
| Interventions |
Intervention characteristics Four‐layer bandage
Short‐stretch bandage
Usual care
|
|
| Outcomes |
Time‐to‐complete wound healing
Proportion of wounds completely healed during follow‐up
Adverse events
Participant health‐related quality of life/health status
Cost effectiveness
Mean pain score
Outcomes that are not considered in this review but reported in trials:
|
|
| Identification |
Publication type/ status (e.g. conference abstract): 2 full papers (Wong 2012; So 2014), retraction record, conference abstract Trial protocol: not described Source of funding: funded by the Health, Welfare and Food Bureau of Hong Kong (HHSRF #404060481) and a scientific grant of Lohmann & Rauscher GmbH & Co KG, Rengsdorf, Germany. Country of origin: Hong Kong Comments: Wong 2012 published in J Vass Surg 2012;55:1376‐85 was retracted by the Journal’s Editor‐in‐Chief because the study has been previously published in J Eur Acad Dermatol Venereol 2012;26:102‐10. Data extracted for this review were based on the paper from J Eur Acad Dermatol Venereol 2012;26:102‐10. Two authors (the leading and correspondence authors) participate in various scientific projects with medical device companies, such as Smith & Nephew and Lohmann & Rauscher. One author is an employee of Lohmann & Rauscher, the company that provided all the study products. Contact information: A. Andriessen. E‐mail: anneke.a@tiscali.nl |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Eligible patients were randomly assigned to either one of the experimental groups (compression treatment) or the control group using a computer generated list after pre‐test measurements were taken” Comment: low risk of bias because of the use of a proper method of generating random sequence |
| Allocation concealment (selection bias) | Unclear risk | Quote: “After confirming eligibility of a patient and obtaining informed consent, the clinical investigator digitally received the information on the allocation of the patient to one of the treatment groups” [based on retracted paper]. Comment: unclear risk of bias because information provided is insufficient for this domain’s judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Outcome group: all outcomes Quote: "participants and interviewers (data collectors) were not informed of the treatment allocation of the study participants for the duration of the study" (Wong 2008b) Quote: "the interveners who performed wound dressing and/or bandaging were not blinded to the treatment groups. Blinding the interveners is not achievable ..." (Wong 2008b) Comment: high risk of bias because in our judgement it is clearly not possible to blind either participants or personnel and a related study (Wong 2008b) suggest that there is non‐blinding of participants |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome group: all outcomes Quote: "This was a double‐blind study, in which the interviewers who were responsible for the pre‐test and post test data collections were not given any information regarding the group to which the participant was assigned" Comment: low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome group: ulcer healing and time to healing Quote: “All 321 patients were included in the survival analysis on ulcer healing” Comment: low risk of bias because of the use of ITT analysis Outcome group: health of quality and pain Quote: “All withdrawn cases were regarded as unsuccessful in terms of treatment and all variables, including size and pain score” Quote: “Forty‐five patients (14%) were withdrawn before the second data collection at week 24.” Comment: unclear risk of bias because completed case data used for qualify of life and pain outcomes and the rate of dropouts is not high |
| Selective reporting (reporting bias) | High risk | Comment: high risk of bias because costs data presented in results ‐ not mentioned in methods ‐ of the retracted paper of Wong 2012 but not presented in the published paper. Additionally, patient’s flow and outcome data presented in Wong (JEADV 2012, 26, 102–110) and the retracted paper (Wong JVS, 2012, 55,1376‐1385) do not match |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias |
4LB: four‐layer bandage; ABPI: ankle:brachial pressure index; IQR: interquartile range; ITT: intention‐to‐treat; QOL: quality of life; SEM: standard error of the mean.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ACTRN12608000599370 | Ineligible intervention |
| ACTRN12613001213730 | Ineligible intervention |
| Adderley 2014 | Ineligible intervention |
| Akesson 2014 | Ineligible study design |
| Allegra 2001 | Ineligible intervention |
| Alvarez 2005 | Ineligible intervention |
| Ashby 2014 | Ineligible intervention |
| Balleste 2002 | Ineligible intervention |
| Bertaux 2010 | Ineligible intervention |
| Blair 1988 | Ineligible intervention |
| Blecken 2005 | Ineligible intervention |
| Bosanquet 1999 | Ineligible intervention |
| Brennan 2010 | Ineligible intervention |
| Brizzio 2010 | Ineligible intervention |
| Callam 1992a | Ineligible intervention |
| Callam 1992b | Ineligible intervention |
| Cameron 1996 | Ineligible intervention |
| Cherry 1998 | Ineligible intervention |
| Colgan 1996 | Ineligible intervention |
| Cordts 1992 | Ineligible intervention |
| CTRI2010091000230 | Ineligible intervention |
| Danielsen 1998 | Ineligible intervention |
| De Abreu 2015 | Ineligible intervention |
| DePalma 1999 | Ineligible intervention |
| Dolibog 2013 | Ineligible intervention |
| Dolibog 2014 | Ineligible intervention |
| Duby 1993 | Ineligible intervention |
| Eriksson 1984b | Ineligible intervention |
| Eriksson 1986 | Ineligible intervention |
| EudraCT2007‐004831‐47 | Ineligible intervention |
| Finlayson 2014 | Ineligible intervention |
| Folguera‐Álvarez 2016 | Ineligible intervention |
| Folguera‐Álvarez 2020 | Ineligible intervention |
| Franek 2014 | Ineligible intervention |
| Franks 1995 | Ineligible intervention |
| Franks 1999 | Ineligible intervention |
| Franks 2000 | Ineligible intervention |
| Franks 2002 | Ineligible intervention |
| Franks 2003 | Ineligible intervention |
| Franks 2004a | Ineligible intervention |
| Franks 2004b | Ineligible intervention |
| Gardon‐Mollard 2003 | Ineligible intervention |
| Gillet 2019 | Ineligible intervention |
| Gould 1993 | Ineligible intervention |
| Gould 1998 | Ineligible intervention |
| Harley 2000 | Ineligible intervention |
| Harley 2004 | Ineligible intervention |
| Harrison 2011 | Ineligible intervention |
| Hendricks 1985 | Ineligible intervention |
| Iglesias 2004 | Ineligible intervention |
| ISRCTN47210331 | Ineligible intervention |
| ISRCTN67751142 | Ineligible intervention |
| Jawien 2008 | Ineligible intervention |
| Jawien 2010 | Ineligible intervention |
| Juenger 2005 | Ineligible intervention |
| Jünger 2004a | Ineligible intervention |
| Jünger 2004b | Ineligible intervention |
| Jünger 2007 | Ineligible intervention |
| Knight 1996 | Ineligible intervention |
| Koksal 2003 | Ineligible intervention |
| Kralj 1997 | Ineligible intervention |
| Kucharzewski 2013 | Ineligible intervention |
| Lazareth 2012 | Ineligible intervention |
| Mancini 2009 | Ineligible intervention |
| Mariani 2008 | Ineligible intervention |
| McCollum 1997 | Ineligible intervention |
| Meyer 2002 | Ineligible intervention |
| Meyer 2003 | Ineligible intervention |
| Milic 2007 | Ineligible intervention |
| Milic 2010 | Ineligible intervention |
| Moffatt 1999 | Ineligible intervention |
| Moffatt 2001 | Ineligible intervention |
| Moffatt 2003a | Ineligible intervention |
| Moffatt 2003b | Ineligible intervention |
| Moffatt 2003c | Ineligible intervention |
| Moffatt 2008 | Ineligible intervention |
| Moffatt 2012 | Ineligible intervention |
| Moody 1999 | Ineligible intervention |
| Mosti 2011 | Ineligible intervention |
| Mosti 2020 | Ineligible intervention |
| NCT00534937 | Ineligible intervention |
| NCT00558662 | Ineligible intervention |
| NCT00821431 | Ineligible intervention |
| NCT02015221 | Ineligible intervention |
| NCT02284373 | Ineligible intervention |
| NCT02364921 | Ineligible intervention |
| NCT02561013 | Ineligible intervention |
| NCT02680834 | Ineligible intervention |
| NCT02728986 | Ineligible intervention |
| NCT02729688 | Ineligible intervention |
| NCT02782689 | Ineligible intervention |
| NCT02790593 | Ineligible intervention |
| NCT02798445 | Ineligible intervention |
| NCT03396731 | Ineligible intervention |
| NCT03404297 | Ineligible population |
| NCT03544788 | Ineligible intervention |
| NCT03736941 | Ineligible study design |
| Nelson 1995 | Ineligible intervention |
| Nelson 2004 | Ineligible intervention |
| Nelson 2007 | Ineligible intervention |
| Northeast 1990 | Ineligible intervention |
| Olofsson 1996 | Ineligible intervention |
| Partsch 1994 | Ineligible intervention |
| Partsch 2001 | Ineligible intervention |
| Polignano 2003 | Ineligible intervention |
| Polignano 2004a | Ineligible intervention |
| Polignano 2004b | Ineligible intervention |
| Price 2008 | Ineligible intervention |
| Robinson 1998 | Ineligible intervention |
| Rocca 2012 | Ineligible intervention |
| Russo 1999 | Ineligible intervention |
| Sabolinski 1996 | Ineligible intervention |
| Scriven 1998 | Ineligible intervention |
| Scriven 2000 | Ineligible study design |
| Smith Nephew 1991 | Ineligible intervention |
| Szewcyzk 2010 | Ineligible intervention |
| Taradaj 2009 | Ineligible intervention |
| Tawfick 2013 | Ineligible intervention |
| Torra i Bou 2003 | Ineligible intervention |
| Travers 1992 | Ineligible intervention |
| Tucker 2008 | Ineligible intervention |
| Ukat 2003 | Ineligible intervention |
| Van Laere 2010 | Ineligible intervention |
| Vowden 2000 | Ineligible intervention |
| Vowden 2001 | Wrong study design |
| Walker 1996 | Ineligible intervention |
| Weller 2012a | Ineligible intervention |
| Wilkinson 1997 | Ineligible intervention |
| Wille 2002 | Ineligible intervention |
| Zuccarelli 1997 | Ineligible intervention |
Characteristics of studies awaiting classification [ordered by study ID]
Cherry 1990.
| Methods | Not available |
| Participants | Not available |
| Interventions | Not available |
| Outcomes | Not available |
| Notes | Unable to obtain full text |
Jünger 2008.
| Methods | Not available |
| Participants | Not available |
| Interventions | Not available |
| Outcomes | Not available |
| Notes | Unable to obtain full text |
Kuznetsov 2009.
| Methods | Not available |
| Participants | Not available |
| Interventions | Not available |
| Outcomes | Not available |
| Notes | Unable to obtain full text |
Robinson 1988.
| Methods | Not available |
| Participants | Not available |
| Interventions | Not available |
| Outcomes | Not available |
| Notes | Unable to obtain full text |
Stacey 2000.
| Methods | Not available |
| Participants | Not available |
| Interventions | Not available |
| Outcomes | Not available |
| Notes | Unable to obtain full text |
Differences between protocol and review
We deleted the outcomes 'ulcer recurrence' and 'participant adherence to compression treatment', as the comparison of recurrence rates after initial healing is observational and the adherence outcome was only relevant to the compression arm of the included studies.
We applied the trial filter developed by Glanville 2019 for the CINAHL Plus search, prepared by the Cochrane Centralised Search Service (CSS), rather than the Scottish Intercollegiate Guidelines Network (SIGN 2019) filter documented in the protocol.
We involved one review author for independent data extraction and another review author to double‐check.
We assessed risk of bias in included studies using the tool with seven specific domains that separated the blinding of participants and personnel from blinding of outcome assessment, rather than a tool with six domains that considered the two domains of blinding together.
We judged overall risk of bias using all seven domains rather than only three domains (sequence generation, allocation concealment, and blinding of outcome assessment) as pre‐planned.
We included mean pain score in our Summary‐of‐findings table together with the other outcomes listed.
Contributions of authors
Chunhu Shi: designed the review; co‐ordinated the review; extracted data; analysed or interpreted data; undertook quality assessment; performed statistical analysis; produced the first draft of the review; contributed to writing or editing the review; wrote to study authors/experts/companies; approved the final review prior to publication.
Jo Dumville: conceived the review; designed the review; co‐ordinated the review; analysed or interpreted data; checked quality of statistical analysis; contributed to writing or editing the review; advised on the review; performed previous work that was the foundation of the current review; approved the final review prior to publication.
Nicky Cullum: conceived the review; designed the review; checked quality of statistical analysis; contributed to writing or editing the review; advised on the review; performed previous work that was the foundation of the current review; approved the final review prior to publication.
Emma Connaughton: checked quality of data extraction; undertook quality assessment; checked quality assessment; contributed to writing or editing the review; approved the final review prior to publication.
Gill Norman: checked quality of data extraction; checked quality assessment; contributed to writing or editing the review; advised on the review; approved the final review prior to publication.
Contributions of the editorial base
Tanya Walsh (Editor): edited the protocol of this review; advised on methodology, interpretation and content; approved the final protocol prior to publication.
Gill Rizzello (Managing Editor): co‐ordinated the editorial process; advised on content; edited the protocol and the review.
Sophie Bishop (Information Specialist): designed the search strategy and edited the search methods section.
Ursula Gonthier (former Editorial Assistant): edited the reference section of the protocol of this review.
Tom Patterson (Editorial Assistant): edited the reference section of the review.
Sources of support
Internal sources
-
Division of Nursing, Midwifery and Social Work, School of Health Sciences, Faculty of Biology, Medicine and Health, University of Manchester, UK
External sources
-
National Institute for Health Research (NIHR), UK
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Wounds. The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care
-
NIHR Manchester Biomedical Research Centre (BRC), UK
This research was co‐funded by the NIHR Manchester BRC. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health and Social Care.
-
National Institute for Health Research Applied Research Collaboration, Greater Manchester, UK
Nicky Cullum and Jo Dumville’s work on this project was partially funded by the National Institute for Health Applied Research Collaboration, Greater Manchester. The views expressed in this publication are those of the authors and not necessarily those of the National Institute for Health Research or the Department of Health and Social Care.
Declarations of interest
Chunhu Shi: I received support from the Tissue Viability Society to attend conferences unrelated to this work. The Doctoral Scholar Awards Scholarship and Doctoral Academy Conference Support Fund (University of Manchester) also supported a PhD and conference attendance respectively; both were unrelated to this work.
Jo Dumville: this research was co‐funded by the National Institute for Health Research Manchester Biomedical Research Centre, and partly funded by the National Institute for Health Research Applied Research Collaboration Greater Manchester.
Nicky Cullum: this research was co‐funded by the National Institute for Health Research Manchester Biomedical Research Centre, and partly funded by the National Institute for Health Research Applied Research Collaboration Greater Manchester.
My previous and current employers received research grant funding from the NHS Research and Development programme and the Health Technology Assessment Programme for systematic reviews on compression, and for two randomised controlled trials of compression. These RCTs were not eligible for inclusion in this review. The funders had no role in the conduct of this review.
Emma Connaughton: none known.
Gill Norman: this research was co‐funded by the National Institute for Health Research Manchester Biomedical Research Centre.
Clifford Richardson (peer reviewer for the protocol for this review) declares that one of the review authors was the Head of the department in which he works, although he himself is not involved with the wound care research team within this department and has not been involved with the preparation or writing of this review.
New
References
References to studies included in this review
Cardoso 2019 {published data only}
- Cardoso LV, De Fátima Guerreiro Godoy M, Czorny RC, De Godoy JM. Using bioelectrical impedance analysis to compare the treatment of edema with the Unna's boot and noncompression in individuals with venous ulcers. Journal of Vascular Nursing 2019;37(1):58-63. [PMID: ] [DOI] [PubMed] [Google Scholar]
Charles 1991 {published data only}
- Charles H. Compression healing of ulcers. Journal of District Nursing 1991;10(3):4-8. [Google Scholar]
- Charles H. Short report: compression healing of venous ulcers. Nursing Times 1992;88(3):52. [PubMed] [Google Scholar]
Daróczy 2006 {published data only}
- Daróczy J. Quality control in chronic wound management: the role of local povidone-iodine (Betadine) therapy. Dermatology: International Journal for Clinical and Investigative Dermatology 2006;212(suppl 1):82-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Eriksson 1984a {published data only}
- Eriksson G, Eklund A-E, Kallings LO. The clinical significance of bacterial growth in venous leg ulcers. Scandinavian Journal of Infectious Diseases 1984;16(2):175-80. [DOI] [PubMed] [Google Scholar]
- Eriksson G, Eklund AE, Liden S, Zetterquist S. Comparison of different treatments of venous leg ulcers: a controlled study using stereophotogrammetry. Current Therapeutic Research 1984;35(4):678-84. [Google Scholar]
Groenewald 1984 {published data only}
- Groenwald JH. Comparative effects of a hydrocolloid dressing and conventional treatment on the healing of venous stasis ulcers. In: Ryan TJ , editors(s). An Environment for Healing: The Role of Occlusion. London (UK): The Royal Society of Medicine, 1984:105-9.
Kikta 1988 {published data only}
- Kikta MJ, Schuler JJ, Meyer JP, Durham JR, Eldrup-Jorgensen J, Schwarcz TH, et al. A prospective, randomized trial of Unna's boots versus hydroactive dressing in the treatment of venous stasis ulcers. Journal of Vascular Surgery 1988;7(3):478-83. [PubMed] [Google Scholar]
Morrell 1998 {published data only}
- Brereton L, Morrell J, Collins K, Walters S, Peters J, Brooker C. Patients' tolerance of leg ulcer treatments. British Journal of Community Health Nursing 1997;2(9):427-30, 432-5. [Google Scholar]
- Collins K, Morrell J, Peters J, Walters S, Brereton L. Variations in venous leg ulcer care in the community. British Journal of Community Nursing 1998;3(1):6-12. [Google Scholar]
- Collins K, Morrell J, Peters J, Walters S, Brooker C, Brereton L. Problems associated with patient satisfaction surveys. British Journal of Community Health Nursing 1997;2(3):156-60. [Google Scholar]
- Morrell CJ, King B, Brereton L. Community-based leg ulcer clinics: organisation and cost-effectiveness. Nursing Times 1998;94(9):51-4. [PubMed] [Google Scholar]
- Morrell CJ, Walters SJ, Dixon S, Collins KA, Brereton LM, Peters J, et al. Cost effectiveness of community leg ulcer clinics: randomised controlled trial. BMJ 1998;316(7143):1487-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrell J, Collins K, Walters S, Brereton L, Dixon S, Peters J, et al. Cost effectiveness of community leg ulcer clinics. Report to NHS Executive Trent. School of Health and Related Research (ScHARR), University of Sheffield 1996.
O'Brien 2003 {published data only}
- Clarke-Moloney M, O'Brien JF, Grace PA, Burke PE. Health-related quality of life during four-layer compression bandaging for venous leg ulcer disease: a randomised controlled trial. Irish Journal of Medical Science 2005;174(2):21-5. [DOI] [PubMed] [Google Scholar]
- O'Brien JF, Grace PA, Perry IJ, Burke PE. Randomised controlled trial: cost-effectiveness of four-layer compression bandaging in venous leg ulcer care. In: 11th Conference of the European Wound Management Association; 2001 May 17-19. Dublin, Ireland, 2001.
- O'Brien JF, Grace PA, Perry IJ, Hannigan A, Clarke-Moloney M, Burke PE. Randomized clinical trial and economic analysis of four-layer compression bandaging for venous ulcers. British Journal of Surgery 2003;90(7):794-8. [DOI] [PubMed] [Google Scholar]
Rubin 1990 {published data only}
- Rubin JR, Alexander J, Plecha EJ, Marman C. Unna's boot vs polyurethane foam dressings for the treatment of venous ulceration. A randomized prospective study. Archives of Surgery 1990;125(4):489-90. [DOI] [PubMed] [Google Scholar]
Taradaj 2007 {published data only}
- Taradaj J, Franek A, Dolibog P, Cierpka L, Blaszczak E. The impact of the sonography and compression therapy on enhancement of healing venous leg ulcers after surgical treatment [Wplyw sono-i kompresoterapil na wspomaganie gojenia owrzodzen zylnych goleni po leczeniu chirurgicznym]. Polski Merkuriusz Lekarski 2007;23(138):426-9. [PubMed] [Google Scholar]
Taylor 1998 {published data only}
- Taylor A, Taylor R, Marcuson R. Comparative healing rates and cost of conventional and four-layer treatment of venous ulcers. Phlebology/Venous Forum of the Royal Society of Medicine 1995;10:85. [Google Scholar]
- Taylor A. A prospective study to compare healing rates and associated treatment costs for current management of venous leg ulcers in the community and a four-layer compression bandage regime. London Macmillan Magazines 1995;1995:199-200. [Google Scholar]
- Taylor AD, Taylor RJ, Marcuson RW. Prospective comparison of healing rates and therapy costs for conventional and four-layer high-compression bandaging treatments of venous leg ulcers. Phlebology 1998;13(1):20-4. [Google Scholar]
Wong 2008a {published data only}
- Wong IK. Chapter 3: Methods. Comparison of Four-Layer Compression Bandage, Short-Stretch Compression Bandage, and Usual Care in the Treatment of Venous Ulcer for Older People in the community (PhD thesis) 2008:63-124.
Wong 2008b {published data only}
- Wong IK, Lee DT, Thompson DR. A randomized controlled trial comparing modern wound management alone or in combination with either short-stretch or long-stretch compression bandages for the treatment of venous ulceration. Phlebologie 2006;35:A54. [Google Scholar]
- Wong IK, Lee DT, Thompson DR. Modern wound care and compression: a randomized controlled study for the comparison of modern wound management only or in combination either with short or long stretch compression bandages in the treatment of venous ulcers. Vasomed 2006;18(4):149-50. [Google Scholar]
- Wong IK. A prospective, randomized-controlled, multi-centre comparison study on 180 leg ulcer patients: modern wound management (MWM) alone versus compression bandages + MWM. In: 16th Conference of the European Wound Management Association; 2006 May 18-20, Prague (Czech Republic). 2006:49, Abstract no.032.
- Wong IK. Comparison of four-layer compression bandage, short-stretch compression bandage, and usual care in the treatment of venous ulcer for older people in the community (PhD thesis). Chinese University of Hong Kong (Hong Kong) 2008:1-375.
Wong 2012 {published data only}
- [No authors listed]. Retracted: randomized controlled trial comparing treatment outcome of two compression bandaging systems and standard care without compression in patients with venous leg ulcers [J Vass Surg 2012;55:1376-85]. Journal of Vascular Surgery 2012;56(6):1830. [PubMed] [Google Scholar]
- Andriessen A, Wong IK, Lee DT, Wong LY, Chao DV, Heung NF, et al. RCT comparing treatment outcome and quality of life of two compression bandaging systems and standard care without compression in venous leg ulcer patients (abstract). EWMA Journal 2011;11(2 Suppl):61. [Google Scholar]
- So WK, Wong IK, Lee DT, Thompson DR, Lau YW, Chao DV, et al. Effect of compression bandaging on wound healing and psychosocial outcomes in older people with venous ulcers: a randomised controlled trial. Hong Kong Medical Journal 2014;20(6 Suppl):40-1. [PubMed] [Google Scholar]
- Wong IK, Andriessen A, Abel M. Clinical and cost efficacy of venous leg ulcer patient treatment: results of a randomized controlled trial comparing two compression bandaging systems and standard care without compression. Phlebology 2012;27(6):311. [Google Scholar]
- Wong IK, Andriessen A, Charles HE, Thompson D, Lee DT, So WKW, et al. Randomized controlled trial comparing treatment outcome of two compression bandaging systems and standard care without compression in patients with venous leg ulcers. Journal of the European Academy of Dermatology and Venereology 2012;26:102-10. [DOI] [PubMed] [Google Scholar]
- Wong IK, Andriessen A, Lee DT, Thompson D, Wong LY, Chao DV, et al. Randomized controlled trial comparing treatment outcome of two compression bandaging systems and standard care without compression in patients with venous leg ulcers. Journal of Vascular Surgery 2012;55(5):1376-85. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
ACTRN12608000599370 {unpublished data only}
- ACTRN12608000599370. A pilot randomised controlled clinical trial to compare the effectiveness of a graduated three layer straight tubular bandaging system when compared to a short stretch compression bandaging system in the management of people with venous ulceration. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=83163 (first received 15 September 2008). [DOI] [PMC free article] [PubMed]
ACTRN12613001213730 {unpublished data only}
- ACTRN12613001213730. Compression therapy in venous leg ulcers. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=365224 (first received 1 November 2013).
Adderley 2014 {published data only}
- Adderley U, Stubbs N. Stockings or bandages for leg-ulcer compression? Nursing Times 2014;110(15):19-20. [PubMed] [Google Scholar]
Akesson 2014 {published data only}
- Akesson N, Oien RF, Forssell H, Fagerström C. Ulcer pain in patients with venous leg ulcers related to antibiotic treatment and compression therapy. British Journal of Community Nursing 2014;Suppl:S6. [DOI] [PubMed] [Google Scholar]
Allegra 2001 {published data only}
- Allegra C, Cariotti R, Bonadeo P, Gasbarro S, Cataldi R, Polignano R. Four-layer compared with Unna's Boot in venous leg ulcer management. 11th Annual Meeting of the European Tissue Repair Society. Cardiff, UK. Wound Repair and Regeneration 2001;9(5):393. [Google Scholar]
- Allegra C, Caritto R, Bonadea P, Gasbarro S, Cataldi R, Polignano R, et al. Four-layer compared with Unna's boot in venous leg ulcer management. 11th European Tissue Repair Society Annual Conference; 2001 September 5-8; Cardiff (Wales) 2001:44.
Alvarez 2005 {published data only}
- Alvarez O, Markowitz L, Booker J. A randomized clinical trial to evaluate healing of chronic venous ulcers in ambulatory patients treated with modified unna's boot and four layer compression bandage. European Wound Management Association Conference; 2005 September 15-17; Stuttgart (Germany) 2005:217; poster abstracts P26.
Ashby 2014 {published data only}
- Ashby RL, Gabe R, Ali S, Adderley U, Bland JM, Cullum NA, et al. Clinical and cost-effectiveness of compression hosiery versus compression bandages in treatment of venous leg ulcers (Venous leg Ulcer Study IV, VenUS IV): a randomised controlled trial. Lancet 2014;383 North American Edition(9920):871-9. [DOI: 10.1016/S0140-6736(13)62368-5] [DOI] [PubMed] [Google Scholar]
- Ashby RL, Gabe R, Ali S, Adderley U, Bland JM, Cullum NA, et al. Clinical efficacy and cost-effectiveness of compression stockings compared with compression bandages in the treatment of venous leg ulcers (Venous Leg Ulcer Study IV, VenUS IV): a randomized, controlled trial [Klinische Wirksamkeit und Kosteneffizienz von Kompressionsstrumpfen im Vergleich zu Kompressionsbandagen bei der Behandlung von venosen Beingeschwuren (Venous leg Ulcer Study IV, VenUS IV): eine randomisierte, kontrollierte Studie]. Vasomed 2015;27(5):268. [Google Scholar]
- Ashby RL, Gabe R, Ali S, Saramago P, Chuang LH, Adderley U. VenUS IV (Venous leg Ulcer Study IV) - compression hosiery compared with compression bandaging in the treatment of venous leg ulcers: a randomised controlled trial, mixed-treatment comparison and decision-analytic model. Health Technology Assessment 2014;18(57):i-xxvii+1-293. [DOI: 10.3310/hta18570] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumville J, Ashby R, Gabe R, Ali S, Saramago P, Adderley U. Venus IV (venous leg ulcer study IV): a randomised controlled trial of compression hosiery versus compression bandaging in the treatment of venous leg ulcers. EWMA Journal 2013;13(1 Suppl):25, abstract no.12. [Google Scholar]
- ISRCTN49373072. VenUS IV: compression hosiery versus compression bandaging in the treatment of venous leg ulcers. www.isrctn.com/ISRCTN49373072 (first received 3 March 2009).
Balleste 2002 {published data only}
- Balleste J, Blanco J, Rueda J, Torra JE, Toda L. Multilayer compressive therapy vs crepe bandage in the treatment of venous ulcers. 12th Conference of the European Wound Management Association; 2002 May 23-25; Granada (Spain) 2002:92.
Bertaux 2010 {published data only}
- Bertaux E, Ettner N. A new approach to treat venous leg ulcers by compression therapy. EWMA Conference; 2010 May 26-28; Geneva (Switzerland) 2010;10:40, Abstract 39. [Google Scholar]
Blair 1988 {published data only}
- Blair SD, Wright DD, Backhouse CM, Riddle E, McCollum CN. Sustained compression and healing of chronic venous ulcers. [erratum appears in BMJ 1988 Dec 10;297(6662): 1500.]. BMJ (Clinical research ed.) 1988;297(6657):1159-61. [DOI: 10.1136/bmj.297.6657.1159] [DOI] [PMC free article] [PubMed] [Google Scholar]
Blecken 2005 {published data only}
- Blecken SR, Villavicencio JL, Kao TC. Comparison of elastic versus nonelastic compression in bilateral venous ulcers: a randomized trial. Journal of Vascular Surgery 2005;42(6):1150-5. [DOI] [PubMed] [Google Scholar]
Bosanquet 1999 {published data only}
- Bosanquet N, Brown D, Straub J, Harper DR, Ruckley CV. Perceived health in a randomised trial of treatment for chronic venous ulceration. European Journal of Vascular and Endovascular Surgery 1999;17(2):155-9. [DOI] [PubMed] [Google Scholar]
Brennan 2010 {published data only}
- Brennan M, Serena TE, Hanft J, Snyder R. Patients with venous leg ulcerations have diminished quality of life: analysis of a multi-center randomized clinical trial. SAWC Spring: the Symposium on Advanced Wounds Care and the Wound Healing Society; 2010 April 17-20; Orlando (FL) 2010:S19, Abstract no: CR-017.
Brizzio 2010 {published data only}
- Brizzio E, Amsler F, Lun B, Blättler W. Comparison of low-strength compression stockings with bandages for the treatment of recalcitrant venous ulcers. Journal of Vascular Surgery 2010;51(2):410-6. [DOI: 10.1016/j.jvs.2009.08.048] [DOI] [PubMed] [Google Scholar]
- Brizzio EO. Comparison of low-strength compression stockings with bandages for the treatment of recalcitrant venous ulcers. A randomized trial. Wound Repair and Regeneration 2010;18(2):A52. [DOI] [PubMed] [Google Scholar]
- Brizzio EO. Comparison of low-strength compression stockings with bandages for the treatment of recalcitrant venous ulcers - a randomized trial. SAWC Spring: the 23rd Annual Symposium on Advanced Wounds Care and the Wound Healing Society; 2010 April 17-20; Orlando (FL) 2010:W65, Abstract No. GP42. [DOI] [PubMed]
Callam 1992a {published data only}
- Callam MJ, Harper DR, Dale JJ, Brown D, Gibson B, Prescott RJ, et al. Lothian and Forth Valley leg ulcer healing trial. Part 1: Elastic versus non-elastic bandaging in the treatment of chronic leg ulceration. Phlebology 1992;7(4):136-41. [Google Scholar]
Callam 1992b {published data only}
- Callam MJ, Harper DR, Dale JJ, Brown D, Gibson B, Prescott RJ, et al. Lothian and Forth Valley leg ulcer healing trial. Part 2: Knitted viscose dressing versus a hydrocellular dressing in the treatment of chronic leg ulceration. Phlebology 1992;7(4):142-5. [Google Scholar]
Cameron 1996 {published data only}
- Cameron J, Hofman D, Poore S, Duby T, Cherry G, Ryan T. A comparative study of two bandage systems. 3rd European Conference on Advances in Wound Management; 1993 October 19-22; Harrogate (UK) 1994:168.
- Cameron J, Hofman D, Poore S, Duby T, Cherry G, Ryan T. A retrospective trial in the treatment of venous leg ulcers. Wounds 1996;8(3):95-100. [Google Scholar]
Cherry 1998 {published data only}
- Cherry GW, Hofman D, Poore S. Tissue repair in patients with chronic venous leg ulcers treated with Prezatide copper acetate gel or standard compression therapy. Wound Repair and Regeneration 1998;6(3):A243. [Google Scholar]
Colgan 1996 {published data only}
- Colgan MP, Teevan M, McBride C, O'Sullivan L, Moore D, Shanik G. Cost comparisons in the management of venous ulceration. Proceedings of the 5th European Conference on Advances in Wound Management; 1995 November 21-24; Harrogate (UK) 1996:103.
Cordts 1992 {published data only}
- Cordts PR, Hanrahan LM, Rodriguez AA, Woodson J, LaMorte WW, Menzoian JO. A prospective, randomized trial of Unna's boot versus Duoderm CGF hydroactive dressing plus compression in the management of venous leg ulcers. Journal of Vascular Surgery 1992;15(3):480-6. [PubMed] [Google Scholar]
CTRI2010091000230 {published data only}
- CTRI/2010/091/000230. A randomized trial of the Flexitouch compression system as an adjunctive treatment for venous stasis ulcer. www.ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=1440&EncHid=&modid=&compid=%27,%271440det%27 (first received 12 March 2010).
Danielsen 1998 {published data only}
- Danielsen L, Madsen M, Henriksen L, Sindrup J, Petersen L. Subbandage pressure measurements comparing a long-stretch with a short-stretch compression bandage. Acta Dermato Venereologica 1998;78(3):201-4. [DOI] [PubMed] [Google Scholar]
- Danielsen L, Madsen SM, Hendriksen L. Venous leg ulcer healing - a randomized prospective study of long-stretch versus short-stretch compression bandages. Phlebology/Venous Forum of the Royal Society of Medicine 1998;13(2):59-63. [Google Scholar]
- Danielsen L, Madsen SM, Henriksen L. Healing of venous leg ulcers. A randomized prospective study of a long-stretch versus short-stretch compression bandage. Ugeskrift for laeger 1999;161(44):6042-5. [PubMed] [Google Scholar]
De Abreu 2015 {published data only}
- De Abreu AM, De Oliveira BG. A study of the Unna Boot compared with the elastic bandage in venous ulcers: a randomized clinical trial. Revista Latino-Americana de Enfermagem 2015;23(4):571-7. [DOI: 10.1590/0104-1169.0373.2590] [DOI] [PMC free article] [PubMed] [Google Scholar]
DePalma 1999 {published data only}
- DePalma RG, Kowallek D, Spence RK, Caprini JA, Nehler MR, Jensen J, et al. Comparison of costs and healing rates of two forms of compression in treating venous ulcers. Vascular Surgery 1999;33(6):683-90. [Google Scholar]
Dolibog 2013 {published data only}
- Dolibog P, Franek A, Taradaj J, Polak A, Blaszczak E, Wcislo L, et al. A randomized, controlled clinical pilot study comparing three types of compression therapy to treat venous leg ulcers in patients with superficial and/or segmental deep venous reflux. Ostomy/Wound Management 2013;59(8):22-30. [PubMed] [Google Scholar]
Dolibog 2014 {published data only}
- Dolibog P, Franek A, Taradaj J, Blaszczak E, Polak A, Brzezinska-Wcislo L, et al. A comparative clinical study on five types of compression therapy in patients with venous leg ulcers. International Journal of Medical Sciences 2014;11(1):34-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Duby 1993 {published data only}
- Duby T, Cherry G, Hoffman D, Cameron J, Doblhoff-Brown D, Ryan T. A randomized trial in the treatment of venous leg ulcers comparing short stretch bandages, four layer bandage system, and a long stretch-paste bandage system. 6th Annual Symposium on Advanced Wound Care 1993.
- Duby T, Hoffman D, Cameron J, Doblhoff-Brown D, Cherry G, Ryan T. A randomized trial in the treatment of venous leg ulcers comparing short stretch bandages, 4 layer bandage system, and a long stretch paste bandage system. Wounds 1993;5(6):276-9. [Google Scholar]
- Duby T, Hoffman D, Cameron J, Dobloff Brown D, Ryan T, Cherry G. A randomised trial in the treatment of venous leg ulcers comparing short stretch bandages, four layer bandage system, and a long stretch-paste bandage system. 2nd European Conference on Advances in Wound Management; 1992 October 20-23; Harrogate (UK) 1993:213-4.
Eriksson 1984b {published data only}
- Eriksson G. Comparative study of hydrocolloid dressings and double layer bandage in treatment of venous stasis ulceration. An Environment for Healing: the Role of Occlusion 1984:111-3.
Eriksson 1986 {published data only}
- Eriksson G. Comparison of two occlusive bandages in the treatment of venous leg ulcers. British Journal of Dermatology 1986;114(2):227-30. [DOI] [PubMed] [Google Scholar]
EudraCT2007‐004831‐47 {published data only}
- EudraCT2007-004831-47. Topical wound oxygen vs. compression therapy in the management of refractory, non-healing venous leg ulcers; a prospective randomised controlled trial. www.clinicaltrialsregister.eu/ctr-search/trial/2007-004831-47/IE (first received 06 September 2007).
Finlayson 2014 {published data only}
- Finlayson KJ, Courtney MD, Gibb MA, O'Brien JA, Parker CN, Edwards HE. The effectiveness of a four-layer compression bandage system in comparison with Class 3 compression hosiery on healing and quality of life in patients with venous leg ulcers: a randomised controlled trial. International Wound Journal 2014;11(1):21-7. [DOI: 10.1111/j.1742-481X.2012.01033.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Folguera‐Álvarez 2016 {published data only}
- Folguera-Álvarez C, Garrido-Elustondo S, Verdú-Soriano J, García-García-Alcalá D, Sanchez-Hernández M, Torres-de Castro OG, et al. ECAMulticapa: effectiveness of double-layered compression therapy for healing venous ulcers in primary care: a study protocol. BMC Nursing 2016;15:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Folguera‐Álvarez 2020 {published data only}
- Folguera-Álvarez C, Garrido-Elustondo S, Rico-Blázquez M, Esparza-Garrido MI, Verdú-Soriano J. Effectiveness of double-layered compression therapy against crepe bandage for healing venous ulcers in primary care: randomized clinical trial. Atencion Primaria 2020;S0212-6567(20):30043-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Franek 2014 {published data only}
- Franek A, Taradaj J, Polak A, Dolibog P, Blaszczak E, Wcislo L, et al. Patients with superficial and/or segmental deep venous reflux: randomized, controlled, clinical pilot study comparing three types of compression therapy for the treatment of venous leg ulcers. Vasomed 2014;26(1):43-6. [PubMed] [Google Scholar]
Franks 1995 {published data only}
- Franks PJ, Bosanquet N, Brown D, Straub J, Harper DR, Ruckley CV. Perceived health in a randomised trial of single and multilayer bandaging for chronic ulceration. Phlebology/Venous Forum of the Royal Society of Medicine 1995;(Suppl 1):17-9.
- Franks PJ, Bosanquet N, Brown D, Straub J, Harper DR, Ruckley CV. Perceived health in a randomised trial of treatment for chronic venous ulceration. European Journal of Vascular and Endovascular Surgery 1999;17(2):155-9. [DOI] [PubMed]
Franks 1999 {published data only}
- Franks PJ, Moffatt CJ, Ellison DA, Connolly M, Fielden S, Groarke L, et al. Quality of life in venous ulceration: a randomized trial of two bandage systems. Phlebology/Venous Forum of the Royal Society of Medicine 1999;14(3):95-9. [DOI: 10.1007/s005230050032] [DOI] [Google Scholar]
Franks 2000 {unpublished data only}
- Franks PJ, Moffatt C. A prospective randomised, factorial, multi-centre open trial to compare the performance of a new foam dressing (Mepilex) with an existing foam dressing (Allevyn non adhesive) and to compare four layer bandaging and short stretch bandaging in the treatment of venous ulceration. Unpublished trial protocol. Trial sponsor: Molnlycke Health Care 2000.
Franks 2002 {published data only}
- Franks PJ, Stevens J, Hourican C, Doherty D, O'Connor T, McCullagh L. Quality of life in venous ulceration: use of the SF-36 in a randomised trial of two bandage systems. 11th Conference of the European Wound Management Association; 2001 May 17-19; Dublin (Ireland) 2001:26.
- Franks PJ, Stevens J, Hourican C, Doherty DC, O'Connor T, McCullagh L, et al. Quality of life in venous ulceration: use of the SF-36 in a randomized trial of two bandage systems (abstract only). Ostomy/Wound Management 2002;48(4):73. [Google Scholar]
Franks 2003 {published data only}
- Franks P, Moody M, Moffatt C. Randomised trial of four layer and cohesive short stretch compression in venous ulceration. Journal of Tissue Viability 2003;13(4):170. [Google Scholar]
Franks 2004a {published data only}
- Franks PJ, Moody M, Moffatt CJ, Martin R, Blewett R, Seymour E, et al. Randomized trial of cohesive short-stretch versus four-layer bandaging in the management of venous ulceration. Wound Repair and Regeneration 2004;12(2):157-62. [DOI: 10.1111/j.1067-1927.2004.012206.x] [DOI] [PubMed] [Google Scholar]
Franks 2004b {published data only}
- Franks PJ, Moody M, Moffatt CJ, Patton J, Bradley L, Chaloner D, et al. Quality of life in a trial of short stretch versus four-layer bandaging in the management of chronic venous ulceration. Phlebology/Venous Forum of the Royal Society of Medicine 2004;19(2):87-91. [Google Scholar]
Gardon‐Mollard 2003 {published data only}
- Gardon-Mollard C, Junger M, Partsch H, Zuccarelli F, Taupin V. Efficacy of a short-stretch tubular compression orthosis compared to a non elastic bandage in the treatment of venous ulcers. 13th Conference of the European Wound Management Association; 2003 May 22-24; Pisa (Italy) 2003:108.
- Gardon-Mollard C, Junger M, Partsch H, Zuccarelli F, Taupin V. Efficacy of a tubular compression orthosis compared to a SSB in the treatment of venous ulcers (abstract from European Venous Forum). Phlebology/Venous Forum of the Royal Society of Medicine 2003;18(3):152. [Google Scholar]
Gillet 2019 {published data only}
- Gillet JL, Guex JJ, Allaert FA, Avouac B, Leger P, Blaise S, et al. Clinical superiority of an innovative two-component compression system versus four-component compression system in treatment of active venous leg ulcers: a randomized trial. Phlebology/Venous Forum of the Royal Society of Medicine 2019;34(9):611-20. [DOI: 10.1177/0268355519833523] [DOI] [PubMed] [Google Scholar]
Gould 1993 {published data only}
- Gould DJ, Campbell S, Harding E. A clinical evaluation of Setopress high compression bandage with Elastocrepe in the management of chronic venous ulceration. 2nd European Conference on Advances in Wound Management; 1992 October 20-23; Harrogate (UK) 1993:211.
- Gould DJ, Campbell S, Harding EF. Short stretch vs long stretch bandages in the management of chronic venous leg ulcers. Phlebology/Venous Forum of the Royal Society of Medicine 1993;8(1):43. [Google Scholar]
Gould 1998 {published data only}
- Gould DJ, Campbell S, Newton H, Duffelen P, Griffin M, Harding EF. Clinical. Setopress vs Elastocrepe in chronic venous ulceration. British Journal of Nursing 1998;7(2):66-73. [DOI] [PubMed] [Google Scholar]
Harley 2000 {published data only}
- Harley J, Harcourt D, Hutchinson B, Liew I. A comparative trial of two layer compression bandaging versus four layer compression bandaging in the treatment of chronic venous ulcers. First World Wound Healing Congress; 2000 September 10-13; Melbourne (Australia) 2000:92.
Harley 2004 {published data only}
- Harley J, Harcourt D, Hutchinson B, McLean M, Long M. A comparative trial of long stretch compression bandaging versus multi-layer compression bandaging in the treatment of chronic venous ulcers. Primary Intention: The Australian Journal of Wound Management 2004;12(1):6-13. [Google Scholar]
Harrison 2011 {published data only}
- Harrison MB. The Canadian bandaging trial: a multi-site RCT of bandaging technologies for venous leg ulcers. The 2011 International Nursing Research Conference; 2011 May 16-18; Harrogate (UK) 2011:50.
- NCT00202267. Community RCT of the effectiveness of two compression bandaging technologies. ClinicalTrials.gov/show/NCT00202267 (first received 20 September 2005).
- Pham B, Harrison MB, Chen MH, Carley ME. Cost-effectiveness of compression technologies for evidence-informed leg ulcer care: results from the Canadian Bandaging Trial. BMC Health Services Research 2012;12(1):346. [DOI: 10.1186/1472-6963-12-346] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hendricks 1985 {published data only}
- Hendricks WM, Swallow RT. Management of statis leg ulcers with Unna's boots versus elastic support stockings. Journal of the American Academy of Dermatology 1985;12(1):90-8. [DOI] [PubMed] [Google Scholar]
Iglesias 2004 {published data only}
- Iglesias C, Nelson EA, Cullum NA, Torgerson DJ. VenUS I: a randomised controlled trial of two types of bandage for treating venous leg ulcers. Health Technology Assessment (Winchester, England) 2004;8(29):iii, 1-105. [DOI] [PubMed] [Google Scholar]
- Iglesias CP, Nelson EA, Cullum N, Torgerson DJ. Economic analysis of VenUS I, a randomized trial of two bandages for treating venous leg ulcers. British Journal of Surgery 2004;91(10):1300-6. [DOI: 10.1002/bjs.4755] [DOI] [PubMed] [Google Scholar]
- Iglesias CP, Nelson EA, Torgerson DJ, Cullulm N. Economic analysis of VenUS I: a randomised controlled trial of two bandages for treating venous leg ulcers. 2nd World Union of Wound Healing Societies Meeting; 2004 July 8-13; Paris (France) 2004:38.
ISRCTN47210331 {published data only}
- ISRCTN47210331. Efficacy and safety of K-Two® versus Actico® compression system in the management of venous leg ulcers: a pilot study. www.isrctn.com/ISRCTN47210331 (first received 20 April 2009).
ISRCTN67751142 {published data only}
- ISRCTN67751142. Community treatment of venous ulcers with concomitant use of compression pump and V.A.C. suction therapy - a novel approach. isrctn.com/ISRCTN67751142 (first received 29 September 2006).
Jawien 2008 {published data only}
- Jawien A, Szewczyk MT, Moscicka P, Cierzniakowska K, Cwajada J. Evaluation of clinical effectiveness of two- and four-layer compression in venous leg ulcer treatment: randomized study. European Venous Forum abstracts. Phlebology/Venous Forum of the Royal Society of Medicine 2008;23:234-5. [Google Scholar]
Jawien 2010 {published data only}
- Jawien A, Szewczyk MT, Banaszkiewicz Z, Moscicka T, Hancke E. Pain associated with venous ulcers and its relation to dynamics of wound healing. EWMA Journal 2010;10(2):42, Abstract 43. [Google Scholar]
Juenger 2005 {published data only}
- Juenger M, Wollina U, Kohnen R, Rabe E. Efficacy of a compression stocking (Venotrain (r) Ulcertec) for therapy of venous leg ulcers. Proceedings of the 15th World Congress International Union of Phlebology; 2005 October 2-7; Rio De Janeiro (Brazil) 2005:177-84.
Jünger 2004a {published data only}
- Jünger M, Partsch H, Ramelet A, Zuccarelli F. Efficacy of a ready-made tubular compression device versus short-stretch compression bandages in the treatment of venous leg ulcers. Wounds 2004;16(10):313-20. [Google Scholar]
- Jünger M, Partsch H, Ramelet AA, Zuccarelli F. Efficacy of a new compression device versus bandages in the treatment of venous leg ulcers. 2nd World Union of Wound Healing Societies Meeting; 2004 July 8-13; Paris (France) 2004:Abstract No.B011.
Jünger 2004b {published data only}
- Jünger M, Wollinar U. Compression therapy of venous leg ulcers with the compression stocking Venotrain® ulcertec: initial data from a prospective randomised study. Vasomed - Bonn 2002;14(4):158-9. [Google Scholar]
- Jünger M, Wollina U, Kohnen R, Rabe E. Efficacy and tolerability of an ulcer compression stocking for therapy of chronic venous ulcer compared with a below-knee compression bandage: results from a prospective, randomized, multicentre trial. Current Medical Research and Opinions 2004;20(10):1613-23. [DOI: 10.1185/030079904X4086] [DOI] [PubMed] [Google Scholar]
- Jünger M, Wollina U, Kohnen R, Rabe E. Efficacy of a compression stocking (Venotrain ® Ulcertec) for therapy of venous leg ulcers. Proceedings of the 15th World Congress International Union of Phlebology; 2005 October; Rio de Janiero (Brazil) 2005.
- Jünger M, Wollina U, Kohnen R, Rabe E. Efficacy of a compression stocking for therapy of chronic venous ulcer compared with bandages. 2nd World Union of Wound Healing Societies Meeting; 2004 July 8-13; Paris (France) 2004:6, Abstract No.A004.
Jünger 2007 {published data only}
- Jünger M, Kohnen R, Stahl HW. Efficacy and cost effectiveness of a compression stocking system for treatment of venous leg ulcers. 17th Conference of the European Wound Management Association; 2007 May 2-4; Glasgow (UK) 2007.
- Jünger M, Kohnen R, Stahl HW. Efficacy and cost effectiveness of a compression stocking system for treatment of venous leg ulcers [Oral presentation no: 24]. 17th Conference of the European Wound Management Association; 2007 May 2-4; Glasgow (UK) 2007:27.
Knight 1996 {published data only}
- Knight CA, McCulloch JM. A comparative study between two compression systems in the treatment of venous insufficiency in leg ulcers. 9th Annual Symposium on Advanced Wound Care and 6th Annual Medical Research Forum on Wound Repair; 1996 April 20-24; Atlanta (GA) 1996:117.
Koksal 2003 {published data only}
- Koksal C, Bozkurt AK. Combination of hydrocolloid dressing and medical compression stocking versus Unna's boot for the treatment of venous leg ulcers. Swiss Medical Weekly 2003;133(25-6):364-8. [DOI] [PubMed] [Google Scholar]
Kralj 1997 {published data only}
- Kralj B, Kosicek M. Randomised comparative trial of single-layer and multi-layer bandages in the treatment of venous leg ulcer. Proceedings of the 6th European Conference on Advances in Wound Management; 1996 October 1-4; Amsterdam (The Netherlands) 1997:158-62.
Kucharzewski 2013 {published data only}
- Kucharzewski M, Kozka M, Urbanek T. Topical treatment of nonhealing venous leg ulcer with propolis ointment. Evidence-Based Complementary & Alternative Medicine: eCAM 2013;2013:254017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lazareth 2012 {published data only}
- Lazareth I, Moffatt C, Dissemond J, Lesne Padieu AS, Truchetet F, Beissert S, et al. Efficacy of two compression systems in the management of VLUs: results of a European RCT. Journal of Wound Care 2012;21(11):553-4, 556, 558. [DOI] [PubMed] [Google Scholar]
Mancini 2009 {published data only}
- Mancini S, Bucalossi M, Mariani F. Elastic stocking* versus bandage. A multicentric randomised trial. EWMA Journal 2009;9(2):Abstract P193. [Google Scholar]
Mariani 2008 {published data only}
- Mariani F, Mattaliano V, Mosti G, Gasbarro V, Bucalossi M, Blattler W, et al. The treatment of venous leg ulcers with a specifically designed compression stocking kit: comparison with bandaging. Phlebologie 2008;37(4):191-7. [Google Scholar]
McCollum 1997 {published data only}
- McCollum CN, Ellison DA, Groake L, Fielden S, Connolly M, Franks PJ. Randomised trial comparing Profore and the original four layer bandage in the treatment of venous leg ulceration. European Wound Management Association Conference; 1997 April 27-29; Milan (Italy) 1997:30.
Meyer 2002 {published data only}
- Meyer FJ, Burnand KG, Lagattolla NR, Eastham D. Randomized clinical trial comparing the efficacy of two bandaging regimens in the treatment of venous leg ulcers. British Journal of Surgery 2002;89(1):40-4. [DOI: 10.1046/j.0007-1323.2001.01936.x] [DOI] [PubMed] [Google Scholar]
- Ruckley CV, Callam MJ, Harper DR, Dale JJ, Gibson B, Prescott RJ, et al. Randomized clinical trial comparing the efficacy of two bandaging regimens in the treatment of venous leg ulcers (Br J Surg 2002; 89: 40-4). Letter 1. British Journal of Surgery 2002;89(6):810; author reply 810. [DOI] [PubMed] [Google Scholar]
- Ruckley CV, Callam MJ, Harper DR, Dale JJ, Gibson B, Prescott RJ, et al. Randomized clinical trial comparing the efficacy of two bandaging regimens in the treatment of venous leg ulcers (Br J Surg 2002; 89: 40-4) [5] (multiple letters). British Journal of Surgery 2002;89(6):810. [DOI] [PubMed] [Google Scholar]
Meyer 2003 {published data only}
- Meyer F, Burnand KG, McGuiness C, Lagattolla N, Eastham D. More venous ulcers are healed by three-layer paste than by four-layer bandages: a randomised, controlled prospective study. Annual Meeting of the Venous Forum of the Royal Society of Medicine; 2000 October 13; Chester (UK) 2000;92-3.
- Meyer FJ, Burnand KG, Lagattolla NR, Eastham D. More venous leg ulcers are healed by three-layer paste than by four-layer bandages: a randomized, controlled, prospective study. British Journal of Surgery 2000;87(Suppl 1):88. [Google Scholar]
- Meyer FJ, Burnand KG, Lagattolla NRF, Eastham D. More venous leg ulcers are healed by three-layer paste than by four-layer bandages: a randomised, controlled, prospective study. Proceedings of the First World Wound Healing Congress; 2000 September 10-13; Melbourne (Australia) 2000:74-5.
- Meyer FJ, McGuinness CL, Lagattolla NRF, Eastham D, Burnand KG. Randomized clinical trial of three-layer paste and four-layer bandages for venous leg ulcers. British Journal of Surgery 2003;90(8):934-40. [DOI] [PubMed] [Google Scholar]
Milic 2007 {published data only}
- Milic DJ, Zivic SS, Bogdanovic DC, Perisic ZD, Milosevic ZD, Jankovic RJ, et al. A randomized trial of the Tubulcus multilayer bandaging system in the treatment of extensive venous ulcers. Journal of Vascular Surgery 2007;46(4):750-5. [DOI: 10.1016/j.jvs.2007.04.062] [DOI] [PubMed] [Google Scholar]
Milic 2010 {published data only}
- Milic DJ, Zivic SS, Bogdanovic DC, Jovanovic MM, Jankovic RJ, Milosevic ZD, et al. The influence of different sub-bandage pressure values on venous leg ulcers healing when treated with compression therapy. Journal of Vascular Surgery 2010;51(3):655-61. [DOI] [PubMed] [Google Scholar]
Moffatt 1999 {published data only}
- Moffatt CJ, Franks PJ, Connolly M, Fielden S, Ellison D, Groake L, et al. Randomised trial comparing two four layer bandage systems in the treatment of venous leg ulceration. Smith and Nephew 1997.
- Moffatt CJ, Simon DA, Franks PJ, Connolly M, Fielden S, Groarke L, et al. Randomised trial comparing two four-layer bandage systems in the management of chronic leg ulceration. Phlebology/Venous Forum of the Royal Society of Medicine 1999;14(4):139-42. [Google Scholar]
Moffatt 2001 {published data only}
- Moffatt CJ, McCullagh L, O'Connor T, Doherty DC, Hourican C, Lewis C, et al. Randomised trial comparing four layer with two layer high compression bandaging in the management of chronic leg ulceration. 12th Conference of the European Wound Management Association; 2002 May 23-25; Granada (Spain) 2002.
- Moffatt CJ, McCullagh L, O'Connor T, Doherty DC, Hourican C, Lewis C. Randomised trial comparing four layer with two layer high compression bandaging in the management of chronic leg ulceration. 11th Conference of the European Wound Management Association; 2001 May 17-19; Dublin (Ireland) 2001:24.
- Moffatt CJ, McCullagh L, O'Connor T, Doherty DC, Hourican C, Stevens J, et al. Randomized trial of four-layer and two-layer bandage systems in the management of chronic venous ulceration. Wound Repair and Regeneration 2003;11(3):166-71. [DOI] [PubMed] [Google Scholar]
Moffatt 2003a {published data only}
- Moffatt CJ, Moody M, Franks PJ. Randomised trial comparing four layer with cohesive short stretch compression bandaging in the management of chronic venous ulceration. 13th Conference of the European Wound Management Association; 2003 May 22-24; Pisa (Italy) 2003:109, presentation 81.
Moffatt 2003b {published data only}
- Moffatt CJ. A multi-centre randomised study comparing a new innovative vari-stretch compression bandaging system with a traditional multi-layer compression bandage system for the management of venous leg ulcers [Abstract]. 13th Conference of the European Wound Management Association; 2003 May 22-24; Pisa (Italy) 2003:111.
Moffatt 2003c {published data only}
- Moffatt C. Results of the examination of proguide in compression therapy (EXPECT) study. Official Satellite Symposium at the European Wound Management Association; 2003 May 23; Pisa (Italy) 2003:8-9.
Moffatt 2008 {published data only}
- Moffatt C, Edwards L, Harding K, Collier M, Treadwell T, Miller M. A randomized, cross-over, clinical trial of a two-layer and four-layer compression bandage system in the treatment of venous leg ulcers. 3rd Congress of the World Union of Wound Healing Societies Meeting; 2008 June 4-8; Toronto (Canada) 2008:Abstract no. OR045.
- Moffatt CJ, Edwards L, Collier M, Treadwell T, Miller M, Shafer L, et al. A randomised controlled 8-week crossover clinical evaluation of the 3M Coban 2 Layer Compression System versus Profore to evaluate the product performance in patients with venous leg ulcers. International Wound Journal 2008;5(2):267-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00301496. Randomized controlled 8-week crossover evaluation of compression bandage systems for venous leg ulcers. ClinicalTrials.gov/show/NCT00301496 (first received 13 March 2006).
Moffatt 2012 {published data only}
- Moffatt C, Lazareth I, Dissemond J, Sauvadet A, Bohbot S. Evaluation of the efficacy and safety of an innovative two-layer (MLB) versus a four-layer (MLB) compression bandage system in the treatment of venous leg ulcers: results of a multicentre European randomized controlled trial (odyssey RCT). EWMA Journal 2012;12(2):107. [Google Scholar]
Moody 1999 {published data only}
- Moody M. Comparison of Rosidal K and SurePress in the treatment of venous leg ulcers. British Journal of Nursing 1999;8(6):345-50. [DOI] [PubMed] [Google Scholar]
Mosti 2011 {published data only}
- Mosti G, Crespi A, Mattaliano V. Comparison between a new, two-component compression system with zinc paste bandages for leg ulcer healing: a prospective, multicenter, randomized, controlled trial monitoring sub-bandage pressures. Wounds 2011;23(5):126-34. [PubMed] [Google Scholar]
Mosti 2020 {published data only}
NCT00534937 {unpublished data only}
- NCT00534937. Flexitouch compression system for venous stasis ulcer. ClinicalTrials.gov/show/NCT00534937 (first received 26 September 2007).
NCT00558662 {unpublished data only}
- NCT00558662. Randomized clinical trial (RCT) to compare the efficacy of Coban 2 versus SSB in the treatment of venous leg ulcers. ClinicalTrials.gov/show/NCT00558662 (first received 15 November 2007).
NCT00821431 {unpublished data only}
- NCT00821431. Compression device versus 4-layer compression system. clinicaltrials.gov/ct2/show/NCT00821431 (first received 13 January 2009).
NCT02015221 {unpublished data only}
- NCT02015221. Evaluation of a dual action pneumatic compression device: patient ease of use and comfort. ClinicalTrials.gov/show/NCT02015221 (first received 19 December 2013).
NCT02284373 {unpublished data only}
- NCT02284373. Randomized clinical trial of pneumatic compression device for the treatment of venous ulcers and lymphedema. ClinicalTrials.gov/show/NCT02284373 (first received 6 November 2011).
NCT02364921 {unpublished data only}
- NCT02364921. Effectiveness of double-layer compression therapy in the healing of chronic venous ulcers in primary health care. ClinicalTrials.gov/show/NCT02364921 (first received 18 February 2015).
NCT02561013 {unpublished data only}
- NCT02561013. A clinical study to assess a compression device in patients with venous leg ulcers. ClinicalTrials.gov/show/NCT02561013 (first received 25 September 2015).
NCT02680834 {unpublished data only}
- NCT02680834. VLU non-inferiority study comparing a dual action pneumatic compression device to multi-layer bandaging. ClinicalTrials.gov/show/NCT02680834 (first received 12 February 2016).
NCT02728986 {unpublished data only}
- NCT02728986. Cost evaluation of venous leg ulcers management. ClinicalTrials.gov/show/NCT02728986 (first received 6 April 2016).
NCT02729688 {unpublished data only}
- NCT02729688. Effectiveness of a pressure indicator guided and a conventional bandaging in treatment of venous leg ulcer. ClinicalTrials.gov/show/NCT02729688 (first received 6 April 2016).
NCT02782689 {unpublished data only}
- NCT02782689. Clinical study to assess efficacy and safety of a new compression system in the management of venous leg ulcers. ClinicalTrials.gov/show/NCT02782689 (first received 25 May 2015).
NCT02790593 {unpublished data only}
- NCT02790593. Juxta-Cures™ versus bandaging for venous ulcers. clinicaltrials.gov/ct2/show/NCT02790593 (first received 6 June 2016).
NCT02798445 {unpublished data only}
- NCT02798445. TAPIRS technique plus adjustable compression system in treatment of venous leg ulcers. ClinicalTrials.gov/show/NCT02798445 (first received 14 June 2016).
NCT03396731 {unpublished data only}
- NCT03396731. Efficacy study for Geko device in VLU patients. ClinicalTrials.gov/show/NCT03396731 (first received 11 January 2018).
NCT03404297 {unpublished data only}
- NCT03404297. Compression therapy for leg ulcers and Kaposi sarcoma in western Kenya. clinicaltrials.gov/show/nct03404297 (first received 19 January 2018).
NCT03544788 {unpublished data only}
- NCT03544788. Evaluation of Cirvo™ mobile compression device for treatment of venous leg ulcers. ClinicalTrials.gov/show/NCT03544788 (first received 4 June 2019).
NCT03736941 {published data only}
- NCT03736941. Impact of venotrain UlcerteC venous compression device in the treatment of venous ulcers in daily practice. ClinicalTrials.gov/show/NCT03736941 (first received 9 November 2018).
Nelson 1995 {published data only}
- Gibson B, Nelson EA, Harper DR, Ruckley CV, Prescott RJ, Dale JJ. A prospective, randomized trial of single-layer and multi-layer bandages in the treatment of venous ulceration. Proceedings of the 5th European Conference on Advances in Wound Management; 1995 November 21-24; Harrogate (UK) 1996:56.
- Nelson EA, Harper DR, Ruckley CV, Perscott RJ, Gibson B, Dale JJ. Single-layer versus four-layer bandaging in the treatment of venous ulcers: a randomized controlled trial [abstract]. British Journal of Surgery 1996;83:563. [Google Scholar]
- Nelson EA, Harper DR, Ruckley CV, Prescott RJ, Gibson B, Dale JJ. A randomised trial of single layer and multi-layer bandages in the treatment of chronic venous ulceration. Phlebology '95: Proceedings of the XII Congress Union Internationale de Phlebologie, London 3-8 September 1995 1995;(Suppl 1):915-6.
- Prescott RJ, Nelson EA, Dale JJ, Harper DR, Ruckley CV. Design of randomized controlled trials in the treatment of leg ulcers: more answers with fewer patients. Phlebology 1998;13(3):107-12. [Google Scholar]
Nelson 2004 {published data only}
- ISRCTN06644918. A randomised controlled trial of two bandages for treating venous leg ulcers. isrctn.com/ISRCTN06644918 (first received 25 April 2003).
- Nelson EA, Iglesias CP, Cullum N, Torgerson DJ, VenUS I collaborators. A randomized controlled trial of four-layer and short-stretch compression bandages for venous leg ulcers (VenUS I). 38th Annual Scientific Meeting of the Vascular Surgical Society of Great Britain and Ireland; 2003 November 26-28; Glasgow (UK) 2003:27.
- Nelson EA, Iglesias CP, Cullum N, Torgerson DJ, VenUS I collaborators. Randomized clinical trial of four-layer and short-stretch compression bandages for venous leg ulcers (VenUS I). British Journal of Surgery 2004;91(10):1292-9. [DOI: 10.1002/bjs.4754] [DOI] [PubMed] [Google Scholar]
Nelson 2007 {published data only}
- Dale JJ, Ruckley CV, Harper DR, Gibson B, Nelson EA, Prescott RJ. A factorial trial of drugs, dressings and bandages in the treatment of leg ulcers. Proceedings of the 5th European Conference on Advances in Wound Management; 1995 November 21-24; Harrogate (UK) 1996:193-4.
- Nelson EA, Prescott RJ, Harper DR, Gibson B, Brown D, Ruckley CV. A factorial, randomized trial of pentoxifylline or placebo, four-layer or single-layer compression, and knitted viscose or hydrocolloid dressings for venous ulcers. Journal of Vascular Surgery 2007;45(1):134-41. [DOI: 10.1016/j.jvs.2006.09.043] [DOI] [PubMed] [Google Scholar]
Northeast 1990 {published data only}
- Northeast AD, Layer GT, Wilson NM, Browse NL, Burnand KG. Increased compression expedites venous ulcer healing. Royal Society of Medicine Venous Forum, 1990 October Meeting 1990.
Olofsson 1996 {published data only}
- Olofsson B, Ljunghall K, Nordin-Bjorklund K, Sorensen S, Leppert J. Two therapeutic models in venous leg ulcers are compared: better results with optimized compression. Lakartidningen 1996;93(51-52):4752-4. [PubMed] [Google Scholar]
Partsch 1994 {published data only}
- Partsch H, Horakova MA. Compression stockings for the treatment of venous leg ulcers [Kompressionsstrumpfe zur behandlung venoser unterschenkelgeschwure]. Wiener Medizinische Wochenschrift 1994;144(10-1):242-9. [PubMed] [Google Scholar]
Partsch 2001 {published data only}
- Partsch H, Damstra RJ, Tazelaar DJ, Schuller-Petrovic S, Velders AJ, De Rooij MJ, et al. Multicentre, randomised controlled trial of four-layer bandaging versus short-stretch bandaging in the treatment of venous leg ulcers. Vasa - Journal of Vascular Diseases 2001;30(2):108-13. [DOI] [PubMed] [Google Scholar]
Polignano 2003 {published data only}
- Polignano R, Bonadeo P, Guarnera G. Compression for venous leg ulcers via bandages or stockings: results of a pilot study comparing the performance of a two-layered compression stocking system with that of short stretch bandage. 13th Conference of the European Wound Management Association; 2003 May 22-24; Pisa (Italy) 2003:112.
Polignano 2004a {published data only}
- Polignano R, Guarnera G, Bonadeo P. Evaluation of SurePress Comfort: a new compression system for the management of venous leg ulcers. Journal of Wound Care 2004;13(9):387-91. [DOI] [PubMed] [Google Scholar]
Polignano 2004b {published data only}
- Polignano R, Bonadeo P, Gasbarro S, Allegra C. A randomised controlled study of four-layer compression versus Unna's Boot for venous ulcers. Journal of Wound Care 2004;13(1):21-4. [DOI] [PubMed] [Google Scholar]
Price 2008 {published data only}
- Price P, Harding K, Moffatt C. Randomized, cross-over, clinical trial to compare the impact of two-layer versus a four-layer compression bandage system in the treatment of venous leg ulcer. EWMA Journal 2008;8(2 (Supp)):75, Abstract no. 112. [Google Scholar]
Robinson 1998 {published data only}
- Robinson C, Santilli SM. Warm-Up Active Wound Therapy: a novel approach to the management of chronic venous stasis ulcers. Journal of Vascular Nursing 1998;16(2):38-42 5. [DOI] [PubMed] [Google Scholar]
Rocca 2012 {published data only}
- Rocca A, Compagna R, De Vito D, Della Corte GA, Bianco T, Amato B. Compression therapy in chronic venous disease. European Surgical Research 2012;49 (3-4):140. [Google Scholar]
Russo 1999 {published data only}
- Russo A. Multi-layer versus two-layer bandage system in the treatment of venous leg ulcers: a comparative study. 9th European Conference on Advances in Wound Management; 1999 November 9-11; Harrogate (UK) 1999:16.
Sabolinski 1996 {published data only}
- Sabolinski M, Borchard G, Mulder G. Cost-benefit analysis of living skin equivalent versus graduated, multi-layered compression therapy for the treatment of venous skin ulcers. Proceedings of the 5th European Conference on Advances in Wound Management; 1995 November 21-24; Harrogate (UK) 1996:107-9.
Scriven 1998 {published data only}
- Scriven JM, Taylor LE, Wood AJ, Bell PR, Naylor AR, London NJ. A prospective randomised trial of four-layer versus short stretch compression bandages for the treatment of venous leg ulcers. Annals of the Royal College of Surgeons of England 1998;80(3):215-20. [PMC free article] [PubMed] [Google Scholar]
Scriven 2000 {published data only}
- Scriven JM, Bello M, Taylor LE, Wood AJ, London NJ. Studies of a new multi-layer compression bandage for the treatment of venous ulceration. Journal of Wound Care 2000;9(3):143-7. [DOI] [PubMed] [Google Scholar]
Smith Nephew 1991 {published data only}
- Smith Nephew. CTR87/29. Final report of a study to compare elastic versus non-elastic bandaging and knitted viscose dressings (Tricotex) versus hydrophilic polyurethane dressings (Allevyn) in the treatment of chronic venous leg ulcers. Volume 1. Smith Nephew 1991.
Szewcyzk 2010 {published data only}
- Szewcyzk MT, Jawien A, Cierzniakowska K, Cwajda-Bialasik J, Moscicka P. Comparison of the effectiveness of compression stockings and layer compression systems in venous ulceration treatment. Archives of Medical Science 2010;6(5):791-9. [DOI: 10.5114/aoms.2010.17097] [DOI] [PMC free article] [PubMed] [Google Scholar]
Taradaj 2009 {published data only}
- Taradaj J, Franek A, Brzezinska-Wcislo L, Blaszczak E, Polak A. Randomized trial of medical compression stockings versus two-layer short-stretch bandaging in the management of venous leg ulcers. Phlebologie 2009;38(4):157-63. [Google Scholar]
Tawfick 2013 {published data only}
- Tawfick WA, Sultan S. Technical and clinical outcome of topical wound oxygen in comparison to conventional compression dressings in the management of refractory nonhealing venous ulcers. Vascular & Endovascular Surgery 2013;47(1):30-7. [DOI: 10.1177/1538574412467684] [DOI] [PubMed] [Google Scholar]
Torra i Bou 2003 {published data only}
- Torra i Bou J, Rueda López J, Blanco Blanco J, Torres Ballester J, Toda Lloret L. Varicose ulcers: the use of a system of multilayered compresses or a crepe bandage? Revista Rol de Enfermería 2003;26(6):59-66. [PubMed] [Google Scholar]
Travers 1992 {published data only}
- Travers JP, Dalziel K, Makin GS. Compression bandaging and venous ulcer healing: assessment of a new one layer bandaging technique. 2nd Joint British-Swedish Angiology Meeting; 1991 September 11-13; RSM London (UK) 1991.
- Travers JP, Dalziel KL, Makin GS. Assessment of a new one-layer adhesive bandaging method in maintaining prolonged limb compression and effects on venous ulcer healing. Phlebology 1992;7(2):59-63. [Google Scholar]
Tucker 2008 {published data only}
- Tucker JA. A prospective, multi-site, randomized, cross-over, clinical trial of a two-layer and a four-layer compression bandage system in the treatment of venous leg ulcers. Journal of Wound, Ostomy, and Continence Nursing 2008;35(3S: Supplement):S71. [Google Scholar]
Ukat 2003 {published data only}
- Ukat A, Konig M, Vanscheidt W, Münter KC. Short-stretch versus multilayer compression for venous leg ulcers: a comparison of healing rates. Journal of Wound Care 2003;12(4):139-43. [DOI: 10.12968/jowc.2003.12.4.26490] [DOI] [PubMed] [Google Scholar]
Van Laere 2010 {published data only}
- Van Laere M. Comparative study between 2 short stretch compression systems. EWMA Journal 2010;10(2):149, Abstract P173. [Google Scholar]
Vowden 2000 {published data only}
- Vowden K. A randomized study comparing compliance, healing and complications from three alternative four-layer bandage regimens in the treatment of venous leg ulcers. 9th European Conference on Advances in Wound Management; 1999 November 9-11; Harrogate (UK) 1999:5.
- Vowden KR, Mason A, Wilkinson D, Vowden P. Comparison of the healing rates and complications of three four-layer bandage regimens. Journal of Wound Care 2000;9(6):269-72. [DOI: 10.12968/jowc.2000.9.6.25992] [DOI] [PubMed] [Google Scholar]
Vowden 2001 {published data only}
- Vowden KR, Wilkinson D, Vowden P. The K-Four bandage system: evaluating its effectiveness on recalcitrant venous leg ulcers. Journal of Wound Care 2001;10(5):182-4. [DOI] [PubMed] [Google Scholar]
Walker 1996 {published data only}
- Walker P, Faria DT. Evaluation of a three-layer bandage system versus a four-layer bandage system in venous leg ulcers. 9th Annual Symposium on Advanced Wound Care and 6th Annual Medical Research Forum on Wound Repair; 1996 April 20-24; Atlanta (GA) 1996:121.
Weller 2012a {published data only}
- Weller C, Evans S, Jolley D, McNeil J. A randomised controlled trial to evaluate elastic versus inelastic compression for the treatment of venous ulcers. EWMA Journal 2011;11(2 Suppl):84. [Google Scholar]
- Weller C, Evans S, Staples M, McNeil J. Improving healing rates with a simple to apply, cost effective tubular bandage system: a randomised controlled trial. EWMA Journal 2012;12(2):325. [Google Scholar]
- Weller CD, Evans S, Reid CM, Wolfe R, McNeil J. Protocol for a pilot randomised controlled clinical trial to compare the effectiveness of a graduated three layer straight tubular bandaging system when compared to a standard short stretch compression bandaging system in the management of people with venous ulceration: 3VSS2008. Trials 2010;11(26):26. [DOI: 10.1186/1745-6215-11-26] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller CD, Evans SM, Staples M, Aldons P, McNeil JJ. Healing venous leg ulcers with three layer tubular compression system: a randomized controlled trial. Wound Repair and Regeneration 2012;20(5):A68. [DOI] [PubMed] [Google Scholar]
- Weller CD, Evans SM, Staples MP, Aldons P, McNeil JJ. Randomized clinical trial of three-layer tubular bandaging system for venous leg ulcers. Wound Repair and Regeneration 2012;20(6):822-9. [DOI: 10.1111/j.1524-475X.2012.00839.x] [DOI] [PubMed] [Google Scholar]
Wilkinson 1997 {published data only}
- Franks P, Moffatt C, Wilkinson E. Two bandaging systems... 'Trial of two bandaging systems for chronic venous ulcers' by Wilkinson et al. Journal of Wound Care 1997;6(8):368-70. [PubMed] [Google Scholar]
- Wilkinson E, Buttfield S, Cooper S, Young E. Randomised controlled trial of two systems of compression bandaging for chronic venous leg ulcers. Unpublished report 1996.
- Wilkinson E, Buttfield S, Cooper S, Young E. Trial of two bandaging systems for chronic venous leg ulcers. Journal of Wound Care 1997;6(7):339-40. [DOI] [PubMed] [Google Scholar]
Wille 2002 {published data only}
- Wille J, Burdge J. Compression therapy versus cultured epidermal autografting for treatment of chronic venous ulcers. 12th Conference of the European Wound Management Association; 2002 May 23-25; Granada (Spain) 2002:246.
Zuccarelli 1997 {published data only}
- Zuccarelli F, Allaert FA. Effectivity of support in the treatment of leg ulcers. A comparative multicentre study of elastic bandage (BIFLEX®) versus extensive bandage. Angeiologie 1997;49(5):15-8. [Google Scholar]
References to studies awaiting assessment
Cherry 1990 {published data only}
- Cherry G. Clinical comparison of a new compression bandage. Nursing Standard 1990;8(8 Special Suppl):8-11. [PubMed] [Google Scholar]
Jünger 2008 {published data only}
- Jünger M, Abel M. Ready-to-wear compression device*: a new successful option for the therapy of venous leg ulcers and other indications. EWMA Journal 2008;8(2):268 (Abstract no. P330). [Google Scholar]
Kuznetsov 2009 {published data only}
- Kuznetsov NA, Rodoman GV, Nikitin VG, Karev MA, Shalaeva TI. The use of current bandages in the treatment of patients with venous trophic ulcers of the skin: clinical and economic aspects. Khirurgiia 2009;11:63-9. [PubMed] [Google Scholar]
Robinson 1988 {published data only}
- Robinson BJ. Randomized comparative trial of Duoderm vs Viscopaste PB7 bandage in the management of venous leg ulceration and cost to the community. ICSS 1988;136:101-4. [Google Scholar]
Stacey 2000 {published data only}
- Stacey MC, Hoskin SE, Vandongen Y, Pearce C. Efficacy and cost effectiveness of compression bandaging. First World Wound Healing Congress; 2000 September 10-13; Melbourne (Australia) 2000:75.
Additional references
Australian Wound Management Association 2011
- Australian Wound Management Association Inc and the New Zealand Wound Care Society. Australian and New Zealand Clinical Practice Guideline for Prevention and Management of Venous Leg Ulcers. Osborne Park (Australia): Cambridge Publishing, 2011. [Google Scholar]
Bafaraj 2014
- Bafaraj MG, Cesko E, Weindorf M, Dissemond J. Chronic leg ulcers as a rare cause for the first diagnosis of epidermolysis bullosa dystrophica. International Wound Journal 2014;11(3):274-7. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Barnsbee 2019
- Barnsbee L, Cheng Q, Tulleners R, Lee X, Brain D, Pacella R. Measuring costs and quality of life for venous leg ulcers. International Wound Journal 2019;16(1):112-21. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bolton 2014
- Bolton LL, Girolami S, Corbett L, Van Rijswijk L. Venous and pressure ulcer guidelines. Ostomy/Wound Management 2014;60(11):24-66. [PMID: ] [PubMed] [Google Scholar]
Brereton 1997
- Brereton L, Morrell J, Collins K, Walters S, Peters J, Brooker C. Patients' tolerance of leg ulcer treatments. British Journal of Community Health Nursing 1997;2(9):427-30, 432-5. [Google Scholar]
Cipriani 2013
- Cipriani A, Higgins JP, Geddes JR, Salanti G. Conceptual and technical challenges in network meta-analysis. Annals of Internal Medicine 2013;159(2):130-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Collins 1997
- Collins K, Morrell J, Peters J, Walters S, Brooker C, Brereton L. Problems associated with patient satisfaction surveys. British Journal of Community Health Nursing 1997;2(3):156-60. [Google Scholar]
Collins 1998
- Collins K, Morrell J, Peters J, Walters S, Brereton L. Variations in venous leg ulcer care in the community. British Journal of Community Nursing 1998;3(1):6-12. [Google Scholar]
Craig 2013
- Craig BM, Pickard AS, Stolk E, Brazier JE. US valuation of the SF-6D. Medical Decision Making 2013;33(6):793-803. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cullum 2001
- Cullum NA, Nelson EA, Fletcher A, Sheldon T. Compression for venous leg ulcers. Cochrane Database of Systematic Reviews 2001, Issue 2. Art. No: CD000265. [DOI: 10.1002/14651858.CD000265] [DOI] [PubMed] [Google Scholar]
Cullum 2016
- Cullum N, Buckley HL, Dumville J, Hall J, Lamb K, Madden MT, et al. Wounds research for patient benefit: a 5 year programme of research. Programme Grants for Applied Research 2016;4(13). [PMID: ] [PubMed]
De Carvalho 2018
- De Carvalho MR, Peixoto BU, Silveira IA, De Oliveria BG. A meta-analysis to compare four-layer to short-stretch compression bandaging for venous leg ulcer healing. Ostomy Wound Management 2018;64(5):30-7. [PubMed] [Google Scholar]
Deeks 2021
- Deeks JJ, Higgins JP, Altman DG (editors). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Eberhardt 2014
- Eberhardt RT, Raffetto JD. Chronic venous insufficiency. Circulation 2014;130(4):333-46. [PMID: ] [DOI] [PubMed] [Google Scholar]
Eldridge 2016
- Eldridge S, Campbell M, Campbell M, Dahota A, Giraudeau B, Higgins J, et al. Revised Cochrane risk of bias tool for randomized trials (RoB 2.0) Additional considerations for cluster-randomized trials. sites.google.com/site/riskofbiastool/welcome/rob-2-0-tool/archive-rob-2-0-cluster-randomized-trials-2016 (accessed 24 June 2019).
Franks 2016
- Franks PJ, Barker J, Collier M, Gethin G, Haesler E, Jawien A, et al. Management of patients with venous leg ulcer: challenges and current best practice. Journal of Wound Care 2016;25(Suppl 1):1-67. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ghauri 2010
- Ghauri AS, Nyamekye IK. Leg ulceration: the importance of treating the underlying pathophysiology. Phlebology/Venous Forum of the Royal Society of Medicine 2010;25(Suppl 1):42-51. [PMID: ] [DOI] [PubMed] [Google Scholar]
Glanville 2019
- Glanville J, Dooley G, Wisniewski S, Foxlee R, Noel-Storr A. Development of a search filter to identify reports of controlled clinical trials within CINAHL Plus. Health Information and Libraries Journal 2019;36(1):73-90. [DOI] [PubMed] [Google Scholar]
Gohel 2018
- Gohel MS, Heatley F, Liu X, Bradbury A, Bulbulia R, Cullum N, et al. A randomized trial of early endovenous ablation in venous ulceration. New England Journal of Medicine 2018;378(22):2105-14. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gray 2018
- Gray TA, Rhodes S, Atkinson RA, Rothwell K, Wilson P, Dumville JC, et al. Opportunities for better value wound care: a multiservice, cross-sectional survey of complex wounds and their care in a UK community population. BMJ Open 2018;22(8):e019440. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Herber 2007
- Herber OR, Schnepp W, Rieger MA. A systematic review on the impact of leg ulceration on patients' quality of life. Health and Quality of Life Outcomes 2007;5:44. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Herdman 2011
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Quality of Life Research 2011;21(10):1727-36. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JP, Altman DG, Sterne JA, (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
Higgins 2021
- Higgins JP, Eldridge S, Li T (editors). Chapter 23: Including variants on randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Hróbjartsson 2012
- Hróbjartsson A, Thomsen AS, Emanuelsson F, Tendal B, Hilden J, Boutron I, et al. Observer bias in randomised clinical trials with binary outcomes: systematic review of trials with both blinded and non-blinded outcome assessors. BMJ 2012;344:e1119. [PMID: ] [DOI] [PubMed] [Google Scholar]
Jockenhöfer 2014
- Jockenhöfer F, Gollnick H, Herberger K, Isbary G, Renner R, Stucker M, et al. Aetiology, comorbidities and cofactors of chronic leg ulcers: retrospective evaluation of 1000 patients from 10 specialised dermatological wound care centers in Germany. International Wound Journal 2016;13(5):821-8. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jull 2012
- Jull AB, Arroll B, Parag V, Waters J. Pentoxifylline for treating venous leg ulcers. Cochrane Database of Systematic Reviews 2012, Issue 12. Art. No: CD001733. [DOI: 10.1002/14651858.CD001733.pub3] [CD001733] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2021
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al. Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLOS Medicine 2009;6(7):e1000100. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marston 2016
- Marston W, Tang J, Kirsner RS, Ennis W. Wound Healing Society 2015 update on guidelines for venous ulcers. Wound Repair and Regeneration 2016;24(1):136-44. [PMID: ] [DOI] [PubMed] [Google Scholar]
Mauck 2014
- Mauck KF, Asi N, Elraiyah TA, Undavalli C, Nabhan M, Altayar O, et al. Comparative systematic review and meta-analysis of compression modalities for the promotion of venous ulcer healing and reducing ulcer recurrence. Journal of Vascular Surgery 2014;60(2 Suppl):71S-90S.e1-2. [DOI] [PubMed] [Google Scholar]
McKenzie 2021
- McKenzie JE, Brennan SE, Ryan RE, Thomson HJ, Johnston RV, Thomas J. Chapter 3: Defining the criteria for including studies and how they will be grouped for the synthesis. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Moffatt 2004
- Moffatt CJ, Franks PJ, Doherty DC, Martin R, Blewett R, Ross F. Prevalence of leg ulceration in a London population. QJM: Monthly Journal of the Association of Physicians 2004;97(7):431-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Moffatt 2007
- Moffatt C (editor). Compression Therapy in Practice. Aberdeen (UK): Wounds-UK Books, 2007. [Google Scholar]
Morrell 1996
- Morrell J, Collins K, Walters S, Brereton L, Dixon S, Peters J, et al. Cost effectiveness of community leg ulcer clinics. Report to NHS Executive Trent. School of Health and Related Research (ScHARR), University of Sheffield 1996.
Mosti 2012
- Mosti G, Iabichella ML, Partsch H. Compression therapy in mixed ulcers increases venous output and arterial perfusion. Journal of Vascular Surgery 2012;55(1):122-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Murad 2017
- Murad MH, Mustafa RA, Schünemann HJ, Sultan S, Santesso N. Rating the certainty in evidence in the absence of a single estimate of effect. Evidence-Based Medicine 2017;22(3):85-7. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nelson 2014
- Nelson EA, Hillman A, Thomas K. Intermittent pneumatic compression for treating venous leg ulcers. Cochrane Database of Systematic Reviews 2014, Issue 5. Art. No: CD001899. [DOI: 10.1002/14651858.CD001899.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Norman 2018
- Norman G, Westby MJ, Rithalia AD, Stubbs N, Soares MO, Dumville JC. Dressings and topical agents for treating venous leg ulcers. Cochrane Database of Systematic Reviews 2018, Issue 6. Art. No: CD012583. [DOI: 10.1002/14651858.CD012583.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Donnell 2014
- O'Donnell TF Jr, Passman MA, Marston WA, Ennis WJ, Dalsing M, Kistner RL, et al. Management of venous leg ulcers: clinical practice guidelines of the Society for Vascular Surgery® and the American Venous Forum. Journal of Vascular Surgery 2014;60(2 Suppl):3S-59S. [PMID: ] [DOI] [PubMed] [Google Scholar]
O'Meara 2012
- O'Meara S, Cullum N, Nelson EA, Dumville JC. Compression for venous leg ulcers. Cochrane Database of Systematic Reviews 2012;11:CD000265. [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Meara 2013
- O'Meara S, Martyn-St James M. Foam dressings for venous leg ulcers. Cochrane Database of Systematic Reviews 2013, Issue 5. Art. No: CD009907. [DOI: 10.1002/14651858.CD009907.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Meara 2015
- O'Meara S, Martyn-St James M, Adderley UJ. Alginate dressings for venous leg ulcers. Cochrane Database of Systematic Reviews 2015, Issue 8. Art. No: CD010182. [DOI: 10.1002/14651858.CD010182.pub3] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Page 2021
- Page MJ, Higgins JP, Sterne JA. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815-34. [PMID: ] [DOI] [PubMed] [Google Scholar]
Partsch 2008
- Partsch H, Clark M, Mosti G, Steinlechner E, Schuren J, Abel M, et al. Classification of compression bandages: practical aspects. Dermatologic Surgery 2008;34(5):600-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Partsch 2011
- Partsch H. Chapter 6: Use of compression therapy. In: Goldman MP, Guex JJ, Weiss RA, editors(s). Sclerotherapy. 5th edition. Edinburgh (UK): W.B. Saunders, 2011:123-55. [Google Scholar]
Partsch 2015
- Partsch H, Mortimer P. Compression for leg wounds. British Journal of Dermatology 2015;173(2):359-69. [PMID: ] [DOI] [PubMed] [Google Scholar]
Persoon 2004
- Persoon A, Heinen MM, Van der Vleuten CJ, De Rooij MJ, Van de Kerkhof PC, Van Achterberg T. Leg ulcers: a review of their impact on daily life. Journal of Clinical Nursing 2004;13(3):341-54. [PMID: ] [DOI] [PubMed] [Google Scholar]
Price 2004
- Price P, Harding K. Cardiff Wound Impact Schedule: the development of a condition-specific questionnaire to assess health-related quality of life in patients with chronic wounds of the lower limb. International Wound Journal 2004;1(1):10-7. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2020 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.4. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Rice 2014
- Rice JB, Desai U, Cummings AK, Birnbaum HG, Skornicki M, Parsons N. Burden of venous leg ulcers in the United States. Journal of Medical Economics 2014;17(5):347-56. [PMID: ] [DOI] [PubMed] [Google Scholar]
Salanti 2012
- Salanti G. Indirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Research Synthesis Methods 2012;3(2):80-97. [PMID: ] [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from guidelinedevelopment.org/handbook.
Schünemann 2021
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
SIGN 2010
- Scottish Intercollegiate Guidelines Network, Healthcare Improvement Scotland. SIGN Guideline 120: Management of chronic venous leg ulcers. www.sign.ac.uk/media/1058/sign120.pdf (accessed 16 June 2021).
SIGN 2019
- Scottish Intercollegiate Guidelines Network (SIGN). Search filters. www.sign.ac.uk/what-we-do/methodology/search-filters/ (accessed 12 June 2019).
Srinivasaiah 2007
- Srinivasaiah N, Dugdall H, Barrett S, Drew PJ. A point prevalence survey of wounds in north-east England. Journal of Wound Care 2007;16(10):413-6, 418-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Thomas 1995
- Thomas S. Compression bandages. In: Cullum N, Roe BH (editors); Leg Ulcers: Nursing Management. London (UK): Scutari Press, 1995:63-74. [Google Scholar]
Thompson 1999
- Thompson SG, Sharp SJ. Explaining heterogeneity in meta-analysis: a comparison of methods. Statistics in Medicine 1999;18(20):2693-708. [PMID: ] [DOI] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vowden 2009a
- Vowden KR, Vowden P. The prevalence, management and outcome for acute wounds identified in a wound care survey within one English health care district. Journal of Tissue Viability 2009;18(1):7-12. [PMID: ] [DOI] [PubMed] [Google Scholar]
Vowden 2009b
- Vowden K, Vowden P, Posnett J. The resource costs of wound care in Bradford and Airedale primary care trust in the UK. Journal of Wound Care 2009;18(3):93-4, 96-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ware 1992
- Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Medical Care 1992;30(6):473-83. [PMID: ] [PubMed] [Google Scholar]
Ware 1996
- Ware J Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Medical Care 1996;34(3):220-33. [PMID: ] [DOI] [PubMed] [Google Scholar]
Weller 2012b
- Weller CD, Ademi Z, Makarounas-Kirchmann K, Stoelwinder J. Economic evaluation of compression therapy in venous leg ulcer randomised controlled trials: a systematic review. Wound Practice & Research 2012;20(1):21-34. [Google Scholar]
Wittens 2015
- Wittens C, Davies AH, Bækgaard N, Broholm R, Cavezzi A, Chastanet S, et al. Management of chronic venous disease: clinical practice guidelines of the European Society for Vascular Surgery (ESVS). European Journal of Vascular and Endovascular Surgery 2015;49(6):678-737. [PMID: ] [DOI] [PubMed] [Google Scholar]
Woo 2013
- Woo KY, Alavi A, Evans R, Despatis M, Allen J. New advances in compression therapy for venous leg ulcers. Surgical Technology International 2013;23:61-8. [PMID: ] [PubMed] [Google Scholar]
References to other published versions of this review
Shi 2019
- Shi C, Dumville JC, Cullum N. Compression bandages or stockings versus no compression for treating venous leg ulcers. Cochrane Database of Systematic Reviews 2019, Issue 8. Art. No: CD013397. [DOI: 10.1002/14651858.CD013397] [DOI] [PMC free article] [PubMed] [Google Scholar]


