Abstract
Recent understanding of the role and contribution of immune cells in disease onset and progression has pioneered the field of immunotherapies. Use of genetic engineering to deliver, correct or enhance immune cells has been clinically successful, especially in the field of cancer immunotherapy. Indeed, one of the most attractive approaches is the introduction of chimeric antigen receptors (CARs) to immune cells, such as T cells. Recent studies revealed that adapting this platform for use in macrophages may widen the spectrum of CAR applications for better control of solid tumors and, thus, extend this treatment strategy to more patients with cancer. Given the novel insights into tumor-associated macrophages and new targeting strategies to boost anticancer therapy, this review aims to provide an overview of the current status of the role of macrophages in cancer therapy. The various genetic engineering approaches that can be used to optimize macrophages for use in oncology are discussed, with special attention dedicated to the implication of the CAR platform on macrophages for anticancer therapy. The current clinical status, challenges and future perspective of macrophage-based drugs are highlighted.
Keywords: receptors, chimeric antigen, macrophages, cell engineering, immunity, innate, immunotherapy
Introduction
Cancer is a very complex and heterogeneous disease, in which a variety of factors contribute to the genetic instability of cancer cells.1 Great progress has been achieved in the field of cancer therapy as a result of the continuous search for anticancer agents with the best efficacy and lowest toxicity. For example, the field of immunotherapy has generated several promising treatment strategies for patients with cancer.2 3 It is well-established that different immune cells play pivotal roles in regulating the tumor microenvironment (TME) by presenting protumor or antitumor functions, such as myeloid-derived suppressor cells, regulatory T cells and macrophages.4 Many current strategies, including use of immune checkpoint inhibitors and cancer vaccines, aim to amplify the natural ability of adaptive immune cells, like the cytotoxic T lymphocytes (CTL), to recognize cancer neoantigens.5 However, this approach is also challenged by the fact that not all tumors display an antigenic component that alerts the immune system. For such tumors, the adoptive transfer of T cells or the transfer of genetically engineered T cells with chimeric antigen receptors (CARs) designed to directly recognize tumor-associated antigens has been suggested.6 Clinical experience to date has clearly shown that the CAR T cell therapy success is more dominant in hematological tumors as compared with solid tumors, which may be due to reduced CAR T cell efficacy to penetrate into solid tumors or to traffic through the inhibitory TME niche.7 However, more clinical data are needed in order to thoroughly assess CAR T cell activity against solid tumors. Of note, it was reported that gamma-delta (γδ) T and natural killer (NK) cells are among the promising sources for developing allogeneic cell therapies due to their unique biological features. The implication of using CARs to increase the cancer recognition capacity of these immune cells was particularly interesting, as γδ T and NK cells are naturally capable of recognizing a broad spectrum of tumor-associated antigens.8 9 In addition, they can target cancer cells in an major histocompatibility complex (MHC)-independent fashion, which reduces the risk of alloreactivity.10 Lastly, due to their interactions with antigen presenting cells, CAR γδ T and NK cells may efficiently bridge the innate and adaptive immune systems to result in optimized immune responses against cancer.10 Nevertheless, the in vivo or ex vivo expansion of these immune cell subsets has hindered more widespread translational application of CAR NK and CAR γδ T cells.11 These potential limitations of CAR T/NK cells highlight the crucial need to develop alternative strategies for use in cancer therapy, such as exploration of other immune cell sources like macrophages. Indeed, it would be highly attractive to exploit the vital immune regulatory properties macrophages present, which range from their activities as professional antigen presenting cells that orchestrate adaptive immune responses, to their abilities to phagocytose and secrete pro-inflammatory cytokines.12 Also, macrophages are abundantly present in the TME of solid tumors via the recruitment of peripheral blood monocytes to solid tumors and subsequent differentiation into tumor-associated macrophages (TAMs).13 14
While many reports showed that TAMs exhibit both M1 and M2 phenotypes, TAMs are often described as M2 macrophages based on the clinical observation that higher M2 macrophage accumulation in the TME is associated with poor prognosis.15
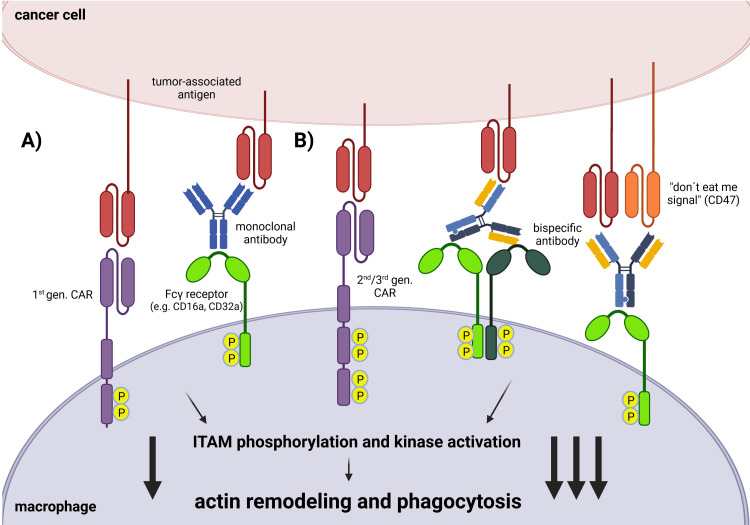
TAMs are considered as key players in the regulation of the TME, where they were mainly shown to promote tumor growth, angiogenesis and metastasis.13 16 17 Hence, numerous approaches aimed to eradicate tumors by targeting TAMs, including depletion, reprograming and repolarization of TAMs or by inhibition of TAM secretion of immune suppressive molecules.18 Nonetheless, the precise role of TAMs in the tumor environment is currently controversially discussed, as TAMs were also reported to be effector cells that recruit CTLs to exert antitumor function.19 Alternatively, other approaches have also emerged to use macrophages in cancer therapy. Instead of directly targeting TAMs, exploitation of unique macrophage properties such as phagocytosis has emerged as a potential therapeutic strategy to engulf cancer cells. There are numerous macrophage-cancer cell interactions that lead to phagocytosis. One prominent strategy that is used in clinical settings is antibody-dependent cellular phagocytosis (ADCP).20 Here, therapeutic antibodies are used to target specific tumor-associated antigens via the Fab region. The internalization of cancer cells is then induced through the binding of Fc receptors (such as CD16a or CD32a) on macrophages via the constant Fc antibody region. The use of synthetically-designed monoclonal antibodies has the advantage of combining the target antigen of interest and the desired macrophage receptor to activate the cells in a specific manner (figures 1A and 2). In addition to Fc receptors, macrophages have additional surface molecules that can trigger phagocytosis (among others, LRP1 or Mac1).21 However, the intracellular signaling pathway that leads to phagocytosis is similar. The surface receptors are coupled to cytoplasmic domains that contain immunoreceptor tyrosine-based activation motifs, which are phosphorylated on receptor binding. The activation of various downstream kinase signaling pathways, such as the MAPK and PI3K/AKT signaling pathways, then leads to actin remodeling and subsequent phagocytosis of the cancer cell (figure 1).21 Another possibility is the use of bispecific antibodies that have dual specificity.20 Here, there are different design strategies. For example, the antibody can either target different tumor antigens or an additional macrophage receptor. Furthermore, phagocytosis can be increased through the binding of phagocytosis checkpoint inhibitors, such as CD47, to block macrophage-inactivating signals. However, the use of antibodies faces several challenges, one of which is the affinity to the inhibitory FC receptor (FcγRIIb) on the surface of macrophages, which negatively regulates effector functions.22 In addition, monoclonal antibody therapy does not distinguish between antitumoral and/or protumoral TAMs.22 Therefore, an alternative strategy to monoclonal antibodies is the adoptive cell therapy with ex vivo genetically-engineered CAR macrophages (CAR-Ms). In order to further strengthen CAR-M functionality (phagocytic activation signal), second or third generation CARs are used to activate additional signaling domains, and thus potentiate additional downstream signaling (figure 1B).23 Among the various strategies to enhance phagocytosis, the modification of macrophages with CAR technology is receiving increasing attention due to the reported success in targeted cancer phagocytosis (figure 3).24 In this review, we aim to provide an updated overview on the various genetic engineering strategies performed on macrophages to optimize their use in oncology. Moreover, special attention is dedicated to the implication of the CAR platform on macrophages, highlighting the current status, challenges and future perspectives.
Figure 1.
Mediation and enhancement of tumor cell phagocytosis. In clinical applications, phagocytosis can be induced in macrophages (A) by using first generation CARs or monoclonal antibodies. (B) To further enhance phagocytosis and increase macrophage activation, second/third generation CARs or bispecific antibodies that show dual specificity for tumor antigens or receptors on macrophages can be used. CAR, chimeric antigen receptor; ITAM, immunoreceptor tyrosine-based activation motif.
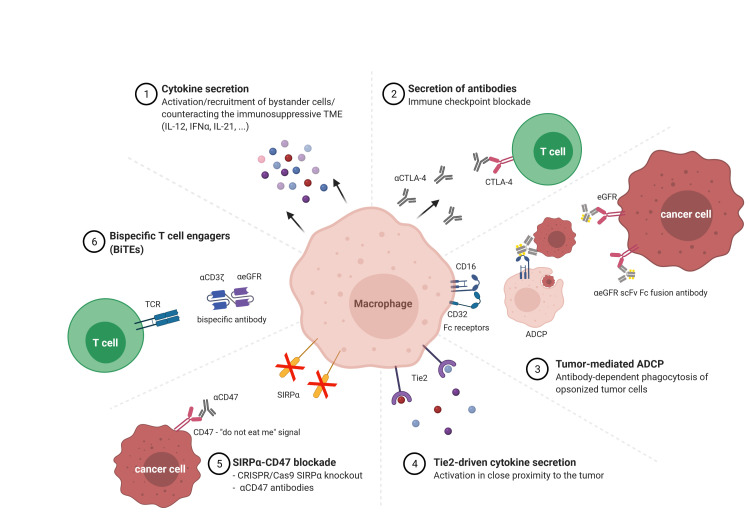
Figure 2.
Diagram of alternative approaches for macrophage immunotherapy. The past decade has shown remarkable progress in immunotherapy using macrophages with various strategies focusing on the activation of endogenous immune cells and the alteration of the immunosuppressive TME. ADCP, antibody-dependent cellular phagocytosis; IFN, interferon; IL, interleukin; TCR, T cell receptor; TME, tumor microenvironment; eGFR, epidermal growth factor receptor; CTLA, cytotoxic T lymphocyte antigen.
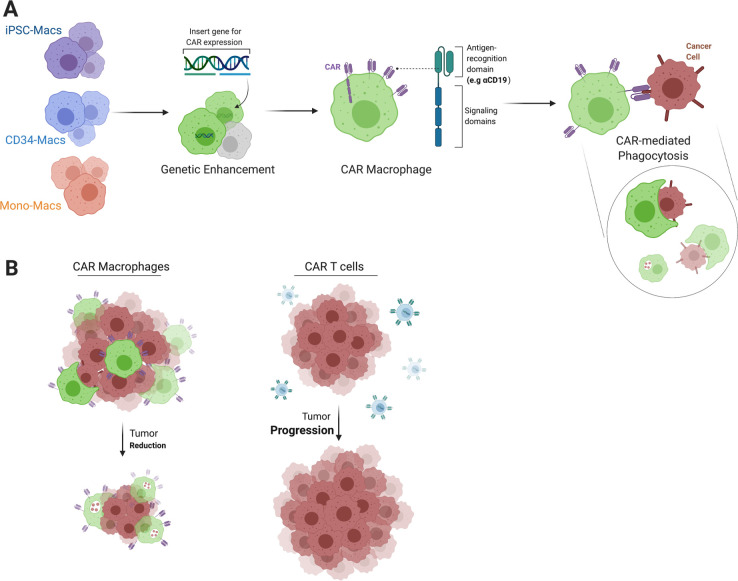
Figure 3.
Recruiting macrophages with CAR constructs gives rise to efficient cancer hunters. Macrophages derived from different sources can be genetically altered to endow enhanced function, for example, with CAR constructs to generate educated macrophages that can target a specific antigen via the CAR antigen recognition domain (A). CAR macrophages display superior homing capacities to solid tumor sites and may better maintain antitumor activity in the tumor microenvironment as compared with CAR T cells, which can lead to enhanced tumor reduction (B). CAR, chimeric antigen receptor.
Targeting TAMs to counteract their protumor function in solid malignancies
Numerous studies were conducted with the goal to identify various approaches to inhibit the tumor supporting roles of TAMs. One strategy aimed to deplete TAMs and inhibit their recruitment to the TME. In fact, a panel of antibodies and small molecule inhibitors that block different macrophage-recruiting chemokines/cytokines such as CCL2, CXCL12, CCL5 and CSF-125–28 are currently under clinical investigation (table 1). Many of these inhibitors showed promising success in phase II clinical trials.29 Targeting the CCL2/CCR2 axis is another useful tactic, as CCL2 is secreted in many tumor subtypes and acts as an essential chemoattractant for monocytes. Hence, the interaction of CCL2 with the CCR2 receptor facilitates macrophage recruitment to the TME, which promotes tumor metastasis and invasion. Sanford et al reported that antagonization of the CCL2/CCR2 axis in pancreatic cancer by CCR2 blockade impaired the recruitment of CCR2 positive monocytes from the bone marrow to the blood, led to TAM depletion, reduced tumor growth, and prevented metastasis in a pancreatic cancer mouse model.25 CSF1/CSF1R is another important signaling pathway that has been successfully targeted with small molecule inhibitors and its inhibition showed efficient TAM depletion. Blockade of the CSF1/CSF1R axis interferes with macrophage activation and survival as the CSF1/CSF1R pathway is important for generation of monocyte progenitors in the bone marrow. Furthermore, CSF1R was reported to regulate the protumor functions of TAMs. Many studies showed CSF1R inhibitors to be particularly useful when combined with chemotherapy in mammary and pancreatic murine models.30 Hence, several candidates are currently evaluated in Phase I/II clinical trials (table 1).
Table 1.
Panel of antibodies and small molecule inhibitors that block different macrophage-recruiting chemokines/cytokines
| Recruiting chemokine | Inhibitor | Cancer type | Phase | Trial design/outcome | NCT number |
| CCL2 | Carlumab | Prostate cancer Solid tumors |
II | Carlumab is well-tolerated with no anticancer activity as a single agent | NCT00992186 |
| CXCL12 | Plerixafor | Metastatic pancreatic cancer | II | Trial is recruiting for a regimen of plerixafor and cemiplimab. Previous preclinical study highlighted the potential of plerixafor in reverting resistance to immune therapy | NCT04177810 |
| CSF‐1 | Emactuzumab | Solid cancers | I | Emactuzumab is tested in combination with atezolizumab, as previous study showed that emactuzumab is well-tolerated and highly active | NCT02323191 |
| CSF-1R | JNJ-40346527 | Solid tumors | I/II | ORR: 1/21 (5%) CBR: 11/21 (52%) |
NCT01572519 |
| CCR2 | CCX872-B | Pancreatic cancer | IB | The combination of CCX872 with FOLFIRINOX chemotherapy resulted in higher OS of 29% | NCT02345408 |
| PF04136309 | Pancreatic cancer | IB | Combination of PF04136309 and FOLFIRINOX resulted in objective tumor response and local tumor control in 97% of patients | NCT01413022 |
CBR, clinical benefit rate; ORR, objective response rate; OS, overall survival.
Additionally, nanoparticle technology was employed in multiple studies to deplete TAMs. For example, Tian et al used calcium bisphosphonate particles to efficiently deplete TAMs upon the intravenous injection of their nanoparticles in murine tumor models. The subsequent effects were reported to be reduced tumor angiogenesis and hypoxia.31 Many of these nanoparticles were also applied in reprograming TAMs to redirect their polarization toward the M1 subtype. Delivery of toll-like receptor (TLR) (7/8) agonist R848, a known potent driver of M1 polarization, by encapsulation in β-cyclodextrin nanoparticles resulted in improved M1 transcription in both murine and human M2 macrophages, illustrating sufficient re-education by the TLR agonist.32 In addition to TLR agonists, the delivery of many other agents and cytokines, like interleukin (IL)-12, and CpG oligonucleotides can induce the transition of the protumor M2 to the antitumor M1 state. Alternatively, reprograming TAMs can be achieved with a CD40 agonist. One study illustrated that combining a CD40 agonist with gemcitabine reduced tumor growth by promoting antitumor macrophages in patients with pancreatic cancer.33 Similarly, transforming growth factor β (TGF-β) is another important molecule that tumor cells use to induce TAM polarization towards the M2 phenotype. TGF-β can promote M2 polarization by different mechanisms, such as induction of IL-1 receptor associated kinase-M expression or by upregulation of Snail expression. In fact, combining a TGF-β blocker with TLR7 activation repolarized TAMs into M1 macrophages and increased tumor apoptosis.34
One of the first reports to describe the simultaneous targeting of TAMs and tumor cells was performed by Ruella and colleagues. In their study, they utilized anti-CD123 CAR T cells, which provided dual targeting of the CD123+ Hodgkin’s lymphoma tumor cells in addition to the CD123+ M2 macrophages within the TAM population. Using this system, anti-CD123 CAR T cells demonstrated efficient eradication of the Hodgkin’s lymphoma in a tumor xenograft mouse model.35
Recently, another interesting approach to selectively deplete M2 TAMs used CAR T cells to target the folate receptor β (FRβ), which is exclusively expressed by immune suppressive macrophages. Rodriguez-Garcia et al, demonstrated that using such CAR T cells in a syngeneic tumor mouse model successfully eliminated the M2 FRβ+ TAMs, enriched the M1 population and induced an influx of tumor-specific CD8+ T cells that halted tumor progression.36
In addition to the described TAM depletion approaches by counteracting TAM recruitment or reprograming TAMs, many recent studies also describe that interference with signaling pathways in TAMs can hamper their cancer supporting functions.37 Interestingly, transcriptome analysis of TAMs from human lung tumor samples demonstrated a significant upregulation of the Wnt/β-catenin signaling pathway, implying its important role in TAM regulation.37 Therefore, Sarode et al tried to deplete β-catenin both pharmacologically and by genetic manipulation of the macrophages, which resulted in reprogramming the M2 macrophages to an M1 phenotype.37 STING is another pathway that was investigated in gastric cancer, as it was noted that STING promotes cancer progression by regulating polarization of TAMs. Knock-down of STING also resulted in repolarization of TAMs towards the pro-inflammatory subtype, similar to the result after depletion of β-catenin.38 Recently, the Hippo/Yap pathway was also reported as one of the pivotal signaling cascades responsible for recruiting TAMs and directly orchestrating their repolarization. However, the detailed mechanism remains to be elucidated, which creates the need for further investigation to pursue whether targeting the Hippo/Yap pathway will result in reprograming and inhibition of TAMs.39
Current pitfalls of targeting TAMs in cancer therapy and the need for alternative approaches
Despite the various studies and the enormous attention dedicated towards targeting TAMs as a promising platform to combat cancer, several challenges remain to be overcome and many questions require investigation to be able to optimize TAMs targeting. One of the challenges stems from the perception and general consideration that the vast majority of macrophages that constitute tumors are the alternative M2 macrophages.40 41 While this might be the case in gastric, colorectal and skin cancer, where TAMs are described to hold protumor roles, the opposite is true in breast, kidney and prostate cancers in which TAMs were reported to have antitumor roles.42 Similarly, TAMs were recently reported to halt tumor growth in medulloblastoma,43 but to support tumor growth in glioblastoma.44 45 The controversial classification of TAMs to date still presents a barrier that hinders the application of therapeutics to target TAMs. The heterogeneous activity of TAMs can be influenced by many factors that vary in every cancer case, such as the tumor location, type, and stage.46 For instance, M1 macrophages are expected to be the dominant subtype in early cancer stages, whereas the transition to M2 occurs in advanced stages.47 Therefore, determining the ideal time to implement TAM targeting protocols is indeed another critical aspect to be considered. In addition, the determination of the origin of TAMs and their precise characterization remain challenging, with several questions yet to be answered. Although many of the TAM targeting agents have reached clinical trials, many of these agents were either discontinued due to insufficient antitumor activity (eg, the anti-CCL2 antibody carlumab), or they were found to have unacceptable toxicity profiles.48 49 In conclusion, it is clear that the principle of targeting cancer by interfering with TAMs has shown some promise, but it must be noted that this success is hindered and limited due to the vital need for deeper phenotypic characterization of these macrophages in each individual case and cancer type.
Bridging CARS from CAR T cell therapy to macrophages
In recent years, T cell-recruiting immunotherapies achieved major clinical success for patients with cancer. Different approaches were implemented to facilitate T cell-mediated killing of malignant cancer cells, such as the bispecific T cell recruiting antibody complex. This is essentially a dual antibody construct composed of a CD3 antibody that binds to T cells via interaction with the T cell receptor (TCR), along with a CD19 antibody that directs the bound T cells to (malignant) B cells.50 This construct was termed as bispecific T cell engager (BiTE). Despite the promising clinical potential of BiTEs, anticancer activity was disappointing in patients with high tumor load.51 Nonetheless, other T cell directed strategies might overcome this challenge. One such strategy is an autologous patient-specific therapy in which the host T cells are collected via leukapheresis and genetically-engineered ex-vivo with CARs prior to reinfusion into the patient.52 CARs contain an extracellular antigen binding domain composed of an antigen recognition domain (eg, a single chain variable fragment (scFv) derived from a monoclonal antibody) that serves to direct the T cell specificity toward binding with a tumor antigen.53 The scFv is linked to a hinge region that provides flexibility to the CAR, a transmembrane domain that transmits the signal on specific binding to the target antigen to the intracellular domain, which is composed of costimulatory and signaling domains (eg, CD3ζ) responsible for CAR T cell activation.53 One of the most common antigens that CAR T cells were programmed to target is the CD19 molecule, because of its high frequency in B cell leukemia and lymphoma, along with the specificity toward the B cell lineage, with no similar expression on other tissues or broader expression pattern relative to other potential markers, like CD22.54–56 Despite the enormous success of CAR T cells in cancer immunotherapy, the clinical application of CAR T cells in solid tumors is less developed, with concerns about the antitumor efficacy and safety (figure 3B). Many studies highlighted that one of the noted obstacles following adoptive transfer of CAR T cells was the inability of these cells to home to the tumor bed, perhaps due to low expression of chemokine receptors to interact with the chemokines secreted by the tumor.57 58 Additionally, it was reported that the infusion of first, second or third generation CAR T cells does not always result in sufficient expansion of those cells, which leads to a lack of response.59 60 Another main concern is the selection of T cells as the therapeutic tool against solid tumors. For example, are these the best immune cells to be employed against solid tumors? Moreover, the widely known phenomenon of ‘T cell exhaustion’ is a topic that has occupied many resources of the scientific community and is a challenge that remains to be fully defined and resolved.61–63 The deficits in T cells were not only linked with CAR T cell activation, but it was also observed that many of the solid tumor patients suffered from intrinsic T cell deficiencies that were attributed to chemotherapy treatment. Das et al reported that the previously accepted T cell threshold for CAR T cells therapy was significantly decreased after chemotherapy administrations.62 Additionally, the depletion of T cells in patients with glioblastoma was found to be unrelated to chemotherapy, but rather due to T cell sequestration in the bone marrow.64 Even if the T cells successfully reached the tumor bed, their inability to adapt to the immune suppressive microenvironment challenges their survival and, thus, their antitumor activity. The combination of downregulation of tumor-associated antigens and an increased expression of checkpoint inhibitors by the cancer cells, in addition to the immunosuppressive cytokines secreted by protumor bystander immune cells, all contribute to fast T cell exhaustion. Furthermore, the severe downregulation of the inflammatory cytokines in the TME hinders the full activation of T cells, which is dependent on the binding of inflammatory cytokines to their receptors on activated T cells that in turn mediate T cell proliferation and differentiation.65 All of these challenges provide strong motivation to broaden the application of the CAR technology to other immune cells that play an important role in the cancer microenvironment, such as macrophages (figure 3B).
Equipping macrophages with CAR constructs: the emerging hope for efficient cancer targeting
It would be very attractive to equip and engineer macrophages with CAR constructs and prepare the modified cells for adoptive transfer to patients with cancer.
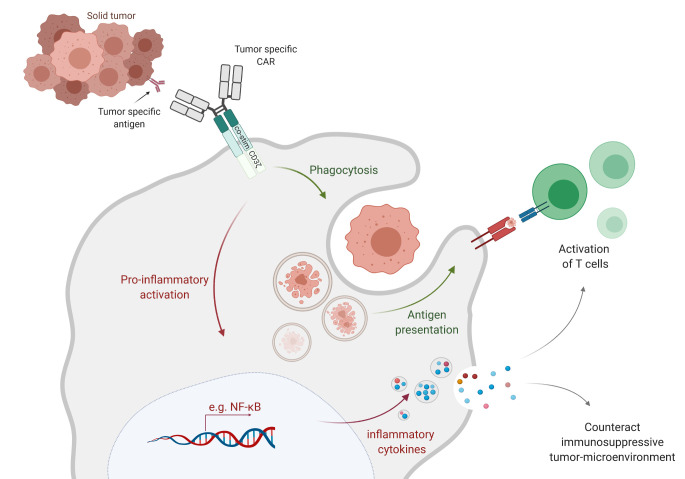
The concept of adoptive transfer of macrophages for cancer therapy was brought to the forefront with many clinical trials.66–68 These trials were performed on several different solid tumors, such as bladder, colorectal, ovarian, gastric and lung cancer. Important evidence for the safety of autologous transfer of up to 3×109 macrophages derived from blood monocytes was acquired, and the process was well-tolerated in different patients with cancer. Nonetheless, the same studies concluded that this approach failed to achieve considerable antitumor efficacy and clinical responses were lacking for many of the conducted trials.69 The perception gained from these reports is that the transferred macrophages require fine tuning and molecular adjustments to acquire better anticancer efficacy. Modern technologies, such as the design of viral vectors or genome editing technologies, allow for the precise modification of cells, including macrophages (figure 3A). However, monocyte/macrophages are particularly characterized by low transduction efficiencies, which can be overcome by the use of, for example, Vpx, an accessory protein that is able to bind to and counteract SAMHD1. Use of Vpx-packaged lentiviral particles for transduction of myeloid cells led to a restored level of dNTPs and, thus, successful reverse transcription of the lentiviral vector constructs.70 71 An additional option for macrophage modification is the use of a specific adenovirus serotype called Ad5F35. Antigen-presenting cells of the myeloid lineage lack the primary receptor for adenoviruses, which results in inefficient transduction with standard Ad5-derived vectors.72 However, CD46 is a complement regulatory protein that is expressed by all nucleated cells and can be used as a receptor for the entry of specific human B adenovirus serotypes. Nilsson and colleagues generated a replication-incompetent chimeric adenoviral vector (Ad5f35) as a method to efficiently transduce malignant hematopoietic cells.72 Klichinsky and colleagues used this strategy and transduced primary human CD14+ peripheral blood monocyte-derived macrophages with an anti-HER2 CAR.24 These anti-HER2 CAR-Ms efficiently induced phagocytosis of the HER2+ ovarian tumor cell line SKOV3. In addition, the CAR-Ms eradicated SKOV3 tumor cells in a dose-dependent manner at a level that directly correlated to CAR expression. In comparison, transduction of macrophages with a control CAR lacked antitumor activity, which showed that the anticancer effect is not affected by transduction.24 Similar findings were translated in vivo in which the SKOV3 tumor burden in NOD-SCID mice was significantly reduced in the group treated with primary human anti-HER2 CAR-Ms. Notably, this work also presented evidence that CAR-Ms can overcome many of the challenges that hamper CAR T cell activity in solid tumors, as CAR-Ms successfully survived and were resistant to the immunosuppressive cytokines present in the TME.24 The infused CAR-Ms were even able to secrete an array of pro-inflammatory cytokines, convert macrophages from an M2 to an M1 phenotype, and direct the tumor niche into a pro-inflammatory environment. Furthermore, Klichinsky et al showed that CAR-Ms were capable of cross presenting NY-ESO-1 antigens to anti-NY-ESO-1 T cells after phagocytosis of NY-ESO-1+ SKOV3 tumor cells. This was marked by CD8+ anti-NY-ESO-1 T cell activation on incubation with both the tumor and CAR-Ms, suggesting epitope spreading by CAR-Ms. In addition, infusion of both CAR-Ms and donor derived T cells showed an increased antitumor response in vivo24 (figure 4). In a follow-up to the previous work, Pierini et al demonstrated the ability of murine bone marrow-derived macrophages to eradicate and phagocytose CT26-HER2+ tumors. Here, infusion of murine derived anti-HER2 CAR-Ms reduced tumor growth, prolonged overall survival and increased intratumoral infiltration of CD4+ and CD8+ T cells, NK cells and dendritic cells.73 Similarly to the findings by Klichinsky et al, Pierini et al showed that CAR-Ms play a pivotal role in regulating the TME via inducing the upregulation of MHC I/II expression on the respective tumor cells, which in turn led to increased tumor-associated antigen presentation to the T cells (figure 4).73
Figure 4.
Closer insight into CAR-macrophage activation and anticancer mechanisms. The binding of tumor antigen with the respective recognition site in the CAR receptor on the surface of CAR-macrophages generates activation signals that mediate tumor phagocytosis, activation of transcription factors such as NF-kB and subsequent release of inflammatory cytokines, which in turn can activate T cell-mediated immunity against the tumor. CAR, chimeric antigen receptor.
In another study, Morrissey et al exploited the unique phagocytosis property of macrophages and focused on that aspect to design new CAR constructs that they called chimeric antigen receptors for phagocytosis (CAR-P).23 The design of CAR-P was similar to that of the classical anti-CD19 CARs used in T cells, with the difference that various murine phagocytic receptors, such as FcRɣ, Bai1, and MerTK, were screened and used as the intracellular signaling domains of the CARs. Their study demonstrated the ability of different CAR-P designs to promote phagocytosis of various cancer-related antigens. Co-culture of CAR-P with the Raji B cancer cell line showed engulfment and reduction of the cancer cell count by over 40%.23
Another attractive design of CAR-Ms aimed to target HER2-expressing cancers while activating the CD147 signaling domain to induce matrix metalloproteinases as a mechanism to destroy the extracellular matrix of the tumor. While in vitro results failed to show strong anti-tumor activity of the CAR-147, infusion of CAR-147 cells into the aggressive 4T1 bearing mouse model showed significant tumor growth inhibition.74 Another study demonstrated a unique tailoring of the CAR-Ms to target CCR7 positive cells, which is an interesting application due to the role of CCR7 in promoting tumor cell metastasis to lymphoid organs. In vivo results highlighted the ability of these CCR7 targeting CAR-Ms to reduce the tumor growth and prolong survival.75 Interestingly, regenerative medicine approaches are expected to provide an attractive solution to overcome the high cost of such personalized approaches by providing a sustainable source of CAR-Ms. Technologies such as delivery of these CARs to induced pluripotent stem cell (iPSC)-derived macrophages may help make these types of therapy available to a greater number of patients. Zhang et al recently showed the impressive potential of generating efficient CAR-Ms from iPSCs, which were capable of reducing tumor growth and activating phagocytosis when tested against leukemia, ovarian and pancreatic cancer cell lines. This was further confirmed in an in vivo mouse model of ovarian cancer, where the tumor burden in the CAR-Ms-treated group was significantly reduced compared with the control group.76 Lastly, Carisma Therapeutics took their successful CAR-M candidate CT-0508 (anti-HER2-CAR-M) a step closer to clinical testing, where it has acquired Food and Drug Administration approval as an investigational new drug. Recently, the first patient was treated in a Phase 1 multicenter clinical trial to test CT-0508 against HER2 positive adenocarcinoma (NCT04660929).
The examples listed in table 2 summarize the preclinical evaluation of CAR-Ms in different cancer subtypes and show promising insight towards the great potential of CAR-Ms.
Table 2.
An overview of the latest studies using CAR-Ms in cancer therapy. Macrophages types, targets, cancer subtypes and significant findings are summarized
| CAR-M target | Macrophage source/type | Tested cancer models (cell lines) | In vivo model | Study findings | Reference | |
| In vitro | In vivo | |||||
| CD19 HER2 Mesothelin |
Human monocytic cell line (THP-1) Human primary macrophages (CD14+ peripheral blood) |
SKOV3 (ovarian cancer) HTB-20 (breast cancer) CRL-2351 (breast cancer) |
NOD/SCID mice bearing SKOV3 | Antigen-specific phagocytosis and tumor clearance | Decreased tumor burden and prolonged overall survival | 24 |
| CD19 CD22 |
Murine bone marrow-derived macrophages (BMDM, C57BL/6J) Murine macrophages (J774A.1) |
Raji B cells | – | Reduced cancer cell number by 40% | – | 23 |
| HER2 | Murine macrophages (Raw264.7 cells) | 4T1 (breast cancer) | BALB /C mice bearing 4T1 | No effect on cancer growth | Significant inhibition of tumor growth | 74 |
| HER2 | Murine bone marrow-derived macrophages | CT-26 (Murine colon carcinoma) AU-565 human breast cancer cells |
BALB /C mice engrafted with CT26-HER2+alone/ with CT-26 wild type | Dose-dependent eradication of AU-565 and CT-26 Cancer cells |
Single tumor model: tumor regression Dual model: Abscopal effect Epitope spreading |
73 |
| CCR7 | Murine macrophages (Raw264.7 cells) | 4T1 (breast cancer) | BALB /C mice bearing 4T1 | Suppressed tumor growth. Prolonged survival |
75 | |
| CD19 Mesothelin |
Induced pluripotent stem cell-derived, CAR-expressing macrophage cells (CAR-iMac) | K562 (chronic myeloid leukemia) OVCAR3 (ovarian cancer) ASPC1 (pancreatic cancer) |
NSG mice bearing ovarian cancer (HO8910) | Antigen-dependent phagocytosis; anticancer cell functions | Tumor burden reduction | 76 |
CAR-Ms, CAR macrophages.
Alternative engineering and molecular reprograming of macrophages into potent cancer eradicating cells
In addition to targeting TAMs or equipping macrophages with CARs as discussed above, the versatile characteristics of macrophages have led to development of other strategies that used genetically engineered macrophages (GEMs) to actively combat tumor cells. One interesting strategy was to use macrophages as cargo vehicles to secrete therapeutic agents locally and constitutively (figure 2). Kaczanowska and colleagues engineered murine hematopoietic stem/progenitor cells (HSPCs) with a lentiviral vector to express the IL-12 transgene.77 The GEMs counteracted the immunosuppressive program in the pre-metastatic niche in a murine rhabdomyosarcoma model through recruitment and activation of the innate and adaptive immune systems. This process led to a decrease in primary tumor burden as well as inhibition of metastases and prolonged survival of tumor-bearing mice. In the same direction, Brempelis and colleagues transduced murine bone marrow-derived macrophages with a lentiviral vector encoding IL-12.78 Brempelis and her group also engineered macrophages that secrete a full-length anti-CTLA-4 antibody over 96 hours to enhance the targeted delivery of checkpoint inhibitors. Interferon (IFN)α was chosen as a secretion molecule by Escobar and her group.79 Here, the focus was set on a specific subpopulation of macrophages: so-called Tie2-expressing macrophages that express the angiopoietin receptor Tie2 only in close proximity to the tumor and thus change to an M2 phenotype with pro-tumoral activity. HSPCs were engineered with a lentiviral vector to express the IFNα transgene driven by a Tie2 promoter to ensure IFNα secretion only in close proximity to the tumor. The GEMs revealed inhibition of primary tumors and lung metastasis in a murine model of breast cancer through recruitment and activation of the innate and adaptive immune systems. Furthermore, a human vector was designed with an improved activation strategy. Since most primitive HSPCs express the Tie2 promoter, which could lead to fast cell exhaustion, transcriptional regulation was ensured by incorporation of miRNA126/130a target sequences. IFNα-expressing human HSPCs transplanted into immunodeficient NOD-SCID-IL2Rγ–/– (NSG) mice resulted in successful microRNA-mediated suppression of transgene expression in immature HSPCs. Escobar and colleagues successfully implemented the same strategy in an immune-competent mouse model that mimics human B-cell acute lymphoblastic leukemia.80 IFNα-secreting HSPCs induced T cell activation and enhanced antitumor activity through counteracting the immunosuppressive signals in the TME. Furthermore, IFNα secretion further improved efficacy of the anti-CTLA-4 checkpoint inhibitor therapy that led to an overall improvement of acute lymphoblastic leukemia inhibition. Catarinella’s group successfully used the same IFNα-Tie2 promoter gene therapy concept to block colorectal cancer colonization of the liver by counteracting the immunosuppressive hepatic microenvironment.81 Another cytokine that is currently used in clinical trials is IL-21, which is physiologically expressed by CD4+ T cells to activate NK and T cells, and is involved in antibody-dependent cell-mediated cytotoxicity.82 An additional strategy that focuses on the successful secretion of cargo proteins via macrophages is the secretion of full-length antibodies. Here, instead of using the CAR technology, Cha and colleagues genetically engineered macrophages to secrete an anti-EGFR scFv-Fc fusion protein to opsonize tumor cells for subsequent phagocytosis by endogenous immune cells. This process is known as ADCP.83 In order to further extend the mechanism of action to recruit and activate bystander immune cells to further improve the antitumor immune response, Gardell and her group engineered human macrophages to secrete a BiTE, which resulted in antigen-dependent T cell responses in a glioblastoma xenograft model.84 In addition, the BiTE-secreting macrophages were equipped with IL-12, which led to increased reduction of tumor burden in both subcutaneous and intracranial mouse glioblastoma models. A similar approach to blocking inhibitory receptors (checkpoints) on T cells is the blockade of the SIRPα-CD47 axis in macrophages. CD47 is the ‘marker of self’ and the ‘do not eat me’ signal and is expressed on almost all cells.85 Binding of CD47 to the inhibitory receptor SIRPα on macrophages leads to the inhibition of the engulfment of self-cells. Cancer cells take advantage of this mechanism and express CD47 in large quantities to protect themselves from the immune surveillance and phagocytic cells. Instead of using antibodies to block the CD47-SIRPα interaction, another approach is to knockout SIRPα on macrophages in order to circumvent the blockade by cancer cells. Ray and colleagues used nanoparticles to deliver the CRISPR/Cas system into RAW264.7 cells to generate SIRPα knockout macrophages.86 Switching off the ‘don’t eat me’ signal resulted in increased phagocytic ability against human osteosarcoma cells. Thus, macrophages are ideal candidates to actively fight cancer as well as to attract additional immune cells to join the combat.
Conclusion
Cancer therapy has recently witnessed an outstanding advancement with the introduction of immunotherapy, where immune cells serve as crucial determinants in both cancer progression and therapy. With deeper and more profound understanding about the roles of these cells in the TME, new forms of immune cell-based therapy have been developed, such as CAR technologies that were applied to generate the clinically successful CAR T cells. Recent insights into CAR T cell applications in cancer highlighted several obstacles that hinder and diminish the full efficacy of these engineered cells. Exploring the potential of CAR macrophages in cancer therapy is particularly intriguing due to the known adaptability of macrophages to the solid tumor niche. In fact, the latest results about CAR macrophages show this strategy to be a very promising novel platform for cancer therapy. Preclinical data showed that CAR macrophages were successfully able to home to an array of solid tumors, including breast, ovarian and pancreatic cancers, with efficient phagocytosis of the solid tumor mass and inhibition of tumor growth both in vitro and in vivo.
Interestingly, due to the versatile properties of macrophages and their promising potential to fight cancer, the field of GEMs is a very prolific and rapidly growing research and clinical area, which includes many other approaches to promote the antitumor function of macrophages in addition to CAR strategies. Nonetheless, with the great expansion of GEMs, the field is lacking critical analyses that compare the different available approaches to modify macrophages in order to determine which strategy results in the greatest inhibition of cancer growth or can be adapted for use against the largest spectrum of cancer subtypes. Another critical question that remains to be addressed is: how efficient are macrophage-based cell therapies when applied as monotherapy to treat cancer? Combination regimens with other forms of cancer immunotherapy may be far more effective to eradicate cancer, especially when one considers the complicated task of overcoming the TME. In fact, in one example Pierini et al demonstrated that the combination of anti PD-1 with HER2 CAR-Ms showed better tumor control and improved the overall survival as compared with monotherapy approaches.73 Hence, careful assessment and further studies are required to determine the optimal synergistic combination of traditional therapy with GEMs. There are currently many macrophage-targeted therapeutics under clinical evaluation (Phase I/II), and the anticipated outcomes of such trials will guide informed decisions regarding which patients can be expected to have the greatest clinical benefit and which of the various strategies (monotherapy/combined) is best suited to which clinical setting.
In summary, the insights learned from the latest advancements in genetically modified macrophages are moving this field a step closer toward the next generation of cell-based anticancer therapy.
Acknowledgments
The figures have been "Created with BioRender.com"
Footnotes
Contributors: SMA, DP, MM, and NL: conception/design, manuscript writing, and final approval of manuscript.
Funding: This work was supported by grants from the German Ministry for Education and Science (iMACnet 01EK1602A), and funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (grant agreement no. 852178). This work was further supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany's Excellence Strategy - EXC 2155 - project number 390874280. The work also received funding from REBIRTH “Förderung aus Mitteln des Niedersächsischen Vorab”, the REBIRTH Center for Translational Regenerative Medicine funded through the State of Lower Saxony (MWK: ZN3440) and the Comprehensive Cancer Center (CCC) Hannover.
Competing interests: NL is author on a pending patent application: 'Stem-cell derived myeloid cells, generation and use thereof', PCT/EP2018/061574.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data sharing not applicable as no data sets generated and/or analyzed for this study. Not applicable.
Ethics statements
Patient consent for publication
Not required.
References
- 1.Podlaha O, Riester M, De S, et al. Evolution of the cancer genome. Trends Genet 2012;28:155–63. 10.1016/j.tig.2012.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang Y, Zhang Z. The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol Immunol 2020;17:807–21. 10.1038/s41423-020-0488-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdin SM, Zaher DM, Arafa E-SA, et al. Tackling cancer resistance by immunotherapy: updated clinical impact and safety of PD-1/PD-L1 inhibitors. Cancers 2018;10. 10.3390/cancers10020032. [Epub ahead of print: 25 01 2018]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smyth MJ, Ngiow SF, Ribas A, et al. Combination cancer immunotherapies tailored to the tumour microenvironment. Nat Rev Clin Oncol 2016;13:143–58. 10.1038/nrclinonc.2015.209 [DOI] [PubMed] [Google Scholar]
- 5.Durgeau A, Virk Y, Corgnac S, et al. Recent advances in targeting CD8 T-cell immunity for more effective cancer immunotherapy. Front Immunol 2018;9:14. 10.3389/fimmu.2018.00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kershaw MH, Westwood JA, Darcy PK. Gene-Engineered T cells for cancer therapy. Nat Rev Cancer 2013;13:525–41. 10.1038/nrc3565 [DOI] [PubMed] [Google Scholar]
- 7.Wagner J, Wickman E, DeRenzo C, et al. Car T cell therapy for solid tumors: bright future or dark reality? Mol Ther 2020;28:2320–39. 10.1016/j.ymthe.2020.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caligiuri MA. Human natural killer cells. Blood 2008;112:461–9. 10.1182/blood-2007-09-077438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edwards SC, Sutton CE, Ladell K, et al. A population of proinflammatory T cells coexpresses αβ and γδ T cell receptors in mice and humans. J Exp Med 2020;217. 10.1084/jem.20190834. [Epub ahead of print: 04 05 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morandi F, Yazdanifar M, Cocco C, et al. Engineering the bridge between innate and adaptive immunity for cancer immunotherapy: focus on γδ T and NK cells. Cells 2020;9:1757. 10.3390/cells9081757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silva-Santos B, Serre K, Norell H. Gammadelta T cells in cancer. Nat Rev Immunol 2015;15:683–91. 10.1038/nri3904 [DOI] [PubMed] [Google Scholar]
- 12.Franken L, Schiwon M, Kurts C. Macrophages: sentinels and regulators of the immune system. Cell Microbiol 2016;18:475–87. 10.1111/cmi.12580 [DOI] [PubMed] [Google Scholar]
- 13.Qian B-Z, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell 2010;141:39–51. 10.1016/j.cell.2010.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noy R, Pollard JW. Tumor-Associated macrophages: from mechanisms to therapy. Immunity 2014;41:49–61. 10.1016/j.immuni.2014.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin Y, Xu J, Lan H. Tumor-Associated macrophages in tumor metastasis: biological roles and clinical therapeutic applications. J Hematol Oncol 2019;12:76. 10.1186/s13045-019-0760-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erreni M, Mantovani A, Allavena P. Tumor-Associated macrophages (TAM) and inflammation in colorectal cancer. Cancer Microenviron 2011;4:141–54. 10.1007/s12307-010-0052-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer 2004;4:71–8. 10.1038/nrc1256 [DOI] [PubMed] [Google Scholar]
- 18.Mantovani A, Marchesi F, Malesci A, et al. Tumour-Associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol 2017;14:399. 10.1038/nrclinonc.2016.217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takeya M, Komohara Y. Role of tumor-associated macrophages in human malignancies: friend or foe? Pathol Int 2016;66:491–505. 10.1111/pin.12440 [DOI] [PubMed] [Google Scholar]
- 20.Weiskopf K, Weissman IL. Macrophages are critical effectors of antibody therapies for cancer. MAbs 2015;7:303–10. 10.1080/19420862.2015.1011450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feng M, Jiang W, Kim BYS, et al. Phagocytosis checkpoints as new targets for cancer immunotherapy. Nat Rev Cancer 2019;19:568–86. 10.1038/s41568-019-0183-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nimmerjahn F, Ravetch JV. Antibodies, Fc receptors and cancer. Curr Opin Immunol 2007;19:239–45. 10.1016/j.coi.2007.01.005 [DOI] [PubMed] [Google Scholar]
- 23.Morrissey MA, Williamson AP, Steinbach AM, et al. Chimeric antigen receptors that trigger phagocytosis. Elife 2018;7:e36688. 10.7554/eLife.36688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klichinsky M, Ruella M, Shestova O, et al. Human chimeric antigen receptor macrophages for cancer immunotherapy. Nat Biotechnol 2020;38:947–53. 10.1038/s41587-020-0462-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanford DE, Belt BA, Panni RZ, et al. Inflammatory monocyte mobilization decreases patient survival in pancreatic cancer: a role for targeting the CCL2/CCR2 axis. Clin Cancer Res 2013;19:3404–15. 10.1158/1078-0432.CCR-13-0525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cassetta L, Pollard JW. Targeting macrophages: therapeutic approaches in cancer. Nat Rev Drug Discov 2018;17:887–904. 10.1038/nrd.2018.169 [DOI] [PubMed] [Google Scholar]
- 27.Pervaiz A, Zepp M, Mahmood S, et al. Ccr5 blockage by maraviroc: a potential therapeutic option for metastatic breast cancer. Cell Oncol 2019;42:93–106. 10.1007/s13402-018-0415-3 [DOI] [PubMed] [Google Scholar]
- 28.Bockorny B, Semenisty V, Macarulla T, et al. BL-8040, a CXCR4 antagonist, in combination with pembrolizumab and chemotherapy for pancreatic cancer: the combat trial. Nat Med 2020;26:878–85. 10.1038/s41591-020-0880-x [DOI] [PubMed] [Google Scholar]
- 29.Butowski N, Colman H, De Groot JF, et al. Orally administered colony stimulating factor 1 receptor inhibitor PLX3397 in recurrent glioblastoma: an ivy Foundation early phase clinical trials Consortium phase II study. Neuro Oncol 2016;18:557–64. 10.1093/neuonc/nov245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitchem JB, Brennan DJ, Knolhoff BL, et al. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res 2013;73:1128–41. 10.1158/0008-5472.CAN-12-2731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tian L, Yi X, Dong Z, et al. Calcium bisphosphonate nanoparticles with chelator-free radiolabeling to deplete tumor-associated macrophages for enhanced cancer radioisotope therapy. ACS Nano 2018;12:11541–51. 10.1021/acsnano.8b06699 [DOI] [PubMed] [Google Scholar]
- 32.Rodell CB, Arlauckas SP, Cuccarese MF, et al. TLR7/8-agonist-loaded nanoparticles promote the polarization of tumour-associated macrophages to enhance cancer immunotherapy. Nat Biomed Eng 2018;2:578–88. 10.1038/s41551-018-0236-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buhtoiarov IN, Lum HD, Berke G, et al. Synergistic activation of macrophages via CD40 and TLR9 results in T cell independent antitumor effects. J Immunol 2006;176:309–18. 10.4049/jimmunol.176.1.309 [DOI] [PubMed] [Google Scholar]
- 34.Peng J, Tsang JYS, Li D, et al. Inhibition of TGF-β signaling in combination with TLR7 ligation re-programs a tumoricidal phenotype in tumor-associated macrophages. Cancer Lett 2013;331:239–49. 10.1016/j.canlet.2013.01.001 [DOI] [PubMed] [Google Scholar]
- 35.Ruella M, Klichinsky M, Kenderian SS, et al. Overcoming the immunosuppressive tumor microenvironment of Hodgkin lymphoma using chimeric antigen receptor T cells. Cancer Discov 2017;7:1154–67. 10.1158/2159-8290.CD-16-0850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodriguez-Garcia A, Lynn RC, Poussin M, et al. Car-T cell-mediated depletion of immunosuppressive tumor-associated macrophages promotes endogenous antitumor immunity and augments adoptive immunotherapy. Nat Commun 2021;12:877. 10.1038/s41467-021-20893-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sarode P, Zheng X, Giotopoulou GA, et al. Reprogramming of tumor-associated macrophages by targeting β-catenin/FOSL2/ARID5A signaling: a potential treatment of lung cancer. Sci Adv 2020;6:eaaz6105. 10.1126/sciadv.aaz6105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miao L, Qi J, Zhao Q, et al. Targeting the sting pathway in tumor-associated macrophages regulates innate immune sensing of gastric cancer cells. Theranostics 2020;10:498. 10.7150/thno.37745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang W, Yang S, Zhang F, et al. Influence of the Hippo-YAP signalling pathway on tumor associated macrophages (TAMs) and its implications on cancer immunosuppressive microenvironment. Ann Transl Med 2020;8:399. 10.21037/atm.2020.02.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Biswas SK, Gangi L, Paul S, et al. A distinct and unique transcriptional program expressed by tumor-associated macrophages (defective NF-kappaB and enhanced IRF-3/STAT1 activation). Blood 2006;107:2112–22. 10.1182/blood-2005-01-0428 [DOI] [PubMed] [Google Scholar]
- 41.Mantovani A, Sozzani S, Locati M, et al. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 2002;23:549–55. 10.1016/s1471-4906(02)02302-5 [DOI] [PubMed] [Google Scholar]
- 42.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res 2006;66:605–12. 10.1158/0008-5472.CAN-05-4005 [DOI] [PubMed] [Google Scholar]
- 43.Maximov V, Chen Z, Wei Y, et al. Tumour-Associated macrophages exhibit anti-tumoural properties in sonic hedgehog medulloblastoma. Nat Commun 2019;10:1–11. 10.1038/s41467-019-10458-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen Z, Feng X, Herting CJ, et al. Cellular and molecular identity of tumor-associated macrophages in glioblastoma. Cancer Res 2017;77:2266–78. 10.1158/0008-5472.CAN-16-2310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang AL, Miska J, Wainwright DA, et al. Ccl2 produced by the glioma microenvironment is essential for the recruitment of regulatory T cells and myeloid-derived suppressor cells. Cancer Res 2016;76:5671–82. 10.1158/0008-5472.CAN-16-0144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu Y, Cao X. The origin and function of tumor-associated macrophages. Cell Mol Immunol 2015;12:1–4. 10.1038/cmi.2014.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tan B, Shi X, Zhang J, et al. Inhibition of Rspo-Lgr4 facilitates checkpoint blockade therapy by switching macrophage polarization. Cancer Res 2018;78:4929–42. 10.1158/0008-5472.CAN-18-0152 [DOI] [PubMed] [Google Scholar]
- 48.Brana I, Calles A, LoRusso PM, et al. Carlumab, an anti-C-C chemokine ligand 2 monoclonal antibody, in combination with four chemotherapy regimens for the treatment of patients with solid tumors: an open-label, multicenter phase 1B study. Target Oncol 2015;10:111–23. 10.1007/s11523-014-0320-2 [DOI] [PubMed] [Google Scholar]
- 49.Sandhu SK, Papadopoulos K, Fong PC, et al. A first-in-human, first-in-class, phase I study of carlumab (CNTO 888), a human monoclonal antibody against CC-chemokine ligand 2 in patients with solid tumors. Cancer Chemother Pharmacol 2013;71:1041–50. 10.1007/s00280-013-2099-8 [DOI] [PubMed] [Google Scholar]
- 50.Riethmüller G. Symmetry breaking: bispecific antibodies, the beginnings, and 50 years on. Cancer Immunity Archive 2012;12. [PMC free article] [PubMed] [Google Scholar]
- 51.Topp MS, Gökbuget N, Stein AS, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol 2015;16:57–66. 10.1016/S1470-2045(14)71170-2 [DOI] [PubMed] [Google Scholar]
- 52.Eshhar Z, Waks T, Gross G, et al. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci U S A 1993;90:720–4. 10.1073/pnas.90.2.720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maher J, Brentjens RJ, Gunset G, et al. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta /CD28 receptor. Nat Biotechnol 2002;20:70–5. 10.1038/nbt0102-70 [DOI] [PubMed] [Google Scholar]
- 54.Kowolik CM, Topp MS, Gonzalez S, et al. Cd28 costimulation provided through a CD19-specific chimeric antigen receptor enhances in vivo persistence and antitumor efficacy of adoptively transferred T cells. Cancer Res 2006;66:10995–1004. 10.1158/0008-5472.CAN-06-0160 [DOI] [PubMed] [Google Scholar]
- 55.Brentjens RJ, Santos E, Nikhamin Y, et al. Genetically targeted T cells eradicate systemic acute lymphoblastic leukemia xenografts. Clin Cancer Res 2007;13:5426–35. 10.1158/1078-0432.CCR-07-0674 [DOI] [PubMed] [Google Scholar]
- 56.Milone MC, Fish JD, Carpenito C, et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol Ther 2009;17:1453–64. 10.1038/mt.2009.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Long KB, Young RM, Boesteanu AC, et al. Car T cell therapy of non-hematopoietic malignancies: detours on the road to clinical success. Front Immunol 2018;9:2740. 10.3389/fimmu.2018.02740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schmidts A, Maus MV. Making CAR T cells a solid option for solid tumors. Front Immunol 2018;9:2593. 10.3389/fimmu.2018.02593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ahmed N, Brawley VS, Hegde M, et al. Human epidermal growth factor receptor 2 (HER2) -specific chimeric antigen receptor-modified T cells for the immunotherapy of HER2-positive sarcoma. J Clin Oncol 2015;33:1688. 10.1200/JCO.2014.58.0225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Heczey A, Louis CU, Savoldo B, et al. Car T cells administered in combination with Lymphodepletion and PD-1 inhibition to patients with neuroblastoma. Mol Ther 2017;25:2214–24. 10.1016/j.ymthe.2017.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ghoneim HE, Fan Y, Moustaki A, et al. De novo epigenetic programs inhibit PD-1 blockade-mediated T cell rejuvenation. Cell 2017;170:e19:142–57. 10.1016/j.cell.2017.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Das RK, Vernau L, Grupp SA, et al. Naïve T-cell deficits at diagnosis and after chemotherapy impair cell therapy potential in pediatric cancers. Cancer Discov 2019;9:492–9. 10.1158/2159-8290.CD-18-1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Leick M, Maus MV. Wishing on a car: understanding the scope of intrinsic T-cell deficits in patients with cancer. Cancer Discov 2019;9:466–8. 10.1158/2159-8290.CD-19-0073 [DOI] [PubMed] [Google Scholar]
- 64.Chongsathidkiet P, Jackson C, Koyama S, et al. Sequestration of T cells in bone marrow in the setting of glioblastoma and other intracranial tumors. Nat Med 2018;24:1459–68. 10.1038/s41591-018-0135-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Labanieh L, Majzner RG, Mackall CL. Programming CAR-T cells to kill cancer. Nat Biomed Eng 2018;2:377–91. 10.1038/s41551-018-0235-9 [DOI] [PubMed] [Google Scholar]
- 66.Lacerna LV, Stevenson GW, Stevenson HC. Adoptive cancer immunotherapy utilizing lymphokine activated killer cells and gamma interferon activated killer monocytes. Pharmacol Ther 1988;38:453–65. 10.1016/0163-7258(88)90014-9 [DOI] [PubMed] [Google Scholar]
- 67.Faradji A, Bohbot A, Schmitt-Goguel M, et al. Phase I trial of intravenous infusion of ex-vivo-activated autologous blood-derived macrophages in patients with non-small-cell lung cancer: toxicity and immunomodulatory effects. Cancer Immunol Immunother 1991;33:319–26. 10.1007/BF01756597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Faradji A, Bohbot A, Frost H, et al. Phase I study of liposomal MTP-PE-activated autologous monocytes administered intraperitoneally to patients with peritoneal carcinomatosis. J Clin Oncol 1991;9:1251–60. 10.1200/JCO.1991.9.7.1251 [DOI] [PubMed] [Google Scholar]
- 69.Andreesen R, Scheibenbogen C, Brugger W, et al. Adoptive transfer of tumor cytotoxic macrophages generated in vitro from circulating blood monocytes: a new approach to cancer immunotherapy. Cancer Res 1990;50:7450–6. [PubMed] [Google Scholar]
- 70.Laguette N, Sobhian B, Casartelli N, et al. Samhd1 is the dendritic- and myeloid-cell-specific HIV-1 restriction factor counteracted by Vpx. Nature 2011;474:654–7. 10.1038/nature10117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bobadilla S, Sunseri N, Landau NR. Efficient transduction of myeloid cells by an HIV-1-derived lentiviral vector that packages the Vpx accessory protein. Gene Ther 2013;20:514–20. 10.1038/gt.2012.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nilsson M, Ljungberg J, Richter J, et al. Development of an adenoviral vector system with adenovirus serotype 35 tropism; efficient transient gene transfer into primary malignant hematopoietic cells. J Gene Med 2004;6:631–41. 10.1002/jgm.543 [DOI] [PubMed] [Google Scholar]
- 73.Pierini S, Gabbasov R, Gabitova L. 132 CAR macrophages (CAR-M) elicit a systemic anti-tumor immune response and synergize with PD1 blockade in immunocompetent mouse models of HER2+ solid tumors. BMJ Specialist Journals 2020. [Google Scholar]
- 74.Zhang W, Liu L, Su H, et al. Chimeric antigen receptor macrophage therapy for breast tumours mediated by targeting the tumour extracellular matrix. Br J Cancer 2019;121:837–45. 10.1038/s41416-019-0578-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Niu Z, Chen G, Chang W, et al. Chimeric antigen receptor-modified macrophages trigger systemic anti-tumour immunity. J Pathol 2021;253:247-257. 10.1002/path.5585 [DOI] [PubMed] [Google Scholar]
- 76.Zhang L, Tian L, Dai X, et al. Pluripotent stem cell-derived CAR-macrophage cells with antigen-dependent anti-cancer cell functions. J Hematol Oncol 2020;13:1–5. 10.1186/s13045-020-00983-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kaczanowska S, Beury DW, Gopalan V, et al. Genetically engineered myeloid cells rebalance the core immune suppression program in metastasis. Cell 2021;184:e21:2033–52. 10.1016/j.cell.2021.02.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brempelis KJ, Cowan CM, Kreuser SA, et al. Genetically engineered macrophages persist in solid tumors and locally deliver therapeutic proteins to activate immune responses. J Immunother Cancer 2020;8. 10.1136/jitc-2020-001356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Escobar G, Gentner B, Naldini L, et al. Engineered tumor-infiltrating macrophages as gene delivery vehicles for interferon-α activates immunity and inhibits breast cancer progression. Oncoimmunology 2014;3:e28696. 10.4161/onci.28696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Escobar G, Barbarossa L, Barbiera G, et al. Interferon gene therapy reprograms the leukemia microenvironment inducing protective immunity to multiple tumor antigens. Nat Commun 2018;9:2896. 10.1038/s41467-018-05315-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Catarinella M, Monestiroli A, Escobar G, et al. Ifnα gene/cell therapy curbs colorectal cancer colonization of the liver by acting on the hepatic microenvironment. EMBO Mol Med 2016;8:155–70. 10.15252/emmm.201505395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Croce M, Rigo V, Ferrini S. Il-21: a pleiotropic cytokine with potential applications in oncology. J Immunol Res 2015;2015:696578. 10.1155/2015/696578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cha EB, Shin KK, Seo J, et al. Antibody-Secreting macrophages generated using CpG-free plasmid eliminate tumor cells through antibody-dependent cellular phagocytosis. BMB Rep 2020;53:442–7. 10.5483/BMBRep.2020.53.8.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gardell JL, Matsumoto LR, Chinn H, et al. Human macrophages engineered to secrete a bispecific T cell engager support antigen-dependent T cell responses to glioblastoma. J Immunother Cancer 2020;8. 10.1136/jitc-2020-001202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Huang C-Y, Ye Z-H, Huang M-Y, et al. Regulation of CD47 expression in cancer cells. Transl Oncol 2020;13:100862. 10.1016/j.tranon.2020.100862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ray M, Lee Y-W, Hardie J, et al. CRISPRed macrophages for cell-based cancer immunotherapy. Bioconjug Chem 2018;29:445–50. 10.1021/acs.bioconjchem.7b00768 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable as no data sets generated and/or analyzed for this study. Not applicable.