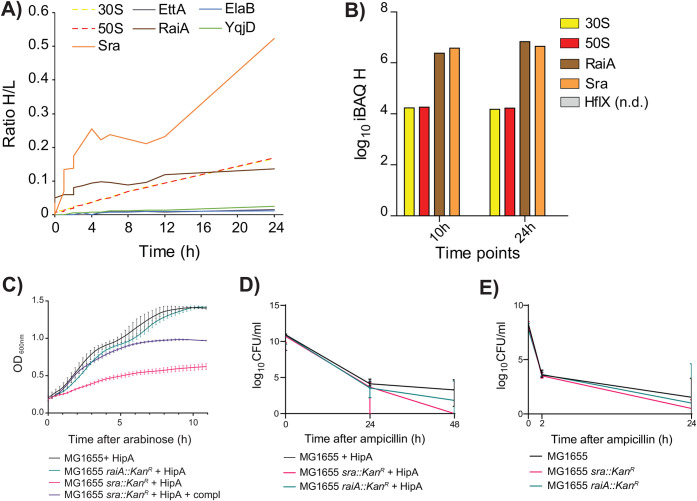
FIG 2.
Ribosome-associated proteins RaiA and Sra show elevated synthesis levels in persisting cells and a role during antibiotic tolerance. (A) All detected ribosomal proteins had comparable label incorporation rates. The synthesis rate of the hibernation factor RaiA was elevated and similar to ribosomal proteins, whereas the synthesis rate of the ribosome-associated protein Sra was markedly higher than that of the ribosomal proteins. Most of the ribosome-associated hibernation factors (e.g., EttA, ElaB, YqjD) showed very low synthesis levels. (B) Estimates of the amounts of newly synthesized proteins based on intensity-based absolute quantification (iBAQ) (25) in the heavy SILAC channel. The amount of newly synthesized RaiA and Sra proteins was about 100 times higher than all ribosomal proteins combined. Depicted are the median values of three biological replicates. n.d., not determined. (C) Wild-type (WT) and ΔraiA deletion strain presented similar growth phenotypes upon HipA ectopic expression, while strain Δsra deletion showed a stronger growth inhibition upon HipA expression, which can be partially complemented by plasmid bearing sra gene with its native promoter. Strains bearing with empty vectors are used as controls. (D) WT strains ectopically expressing HipA were more viable compared to the same strain bearing the empty vector. Two deletion strains (Δsra and ΔraiA) expressing hipA showed a strong decrease in CFU, indicating a potential role of Sra and RaiA in the antibiotic tolerance mechanism. Control experiments including expression of empty vector are presented in Fig. S1H in the supplemental material. Equivalent experiments in the M9 medium are shown in Fig. S1I. (E) Without HipA ectopic overexpression, only the mutant lacking the sra gene showed a lower survival in the LB medium.