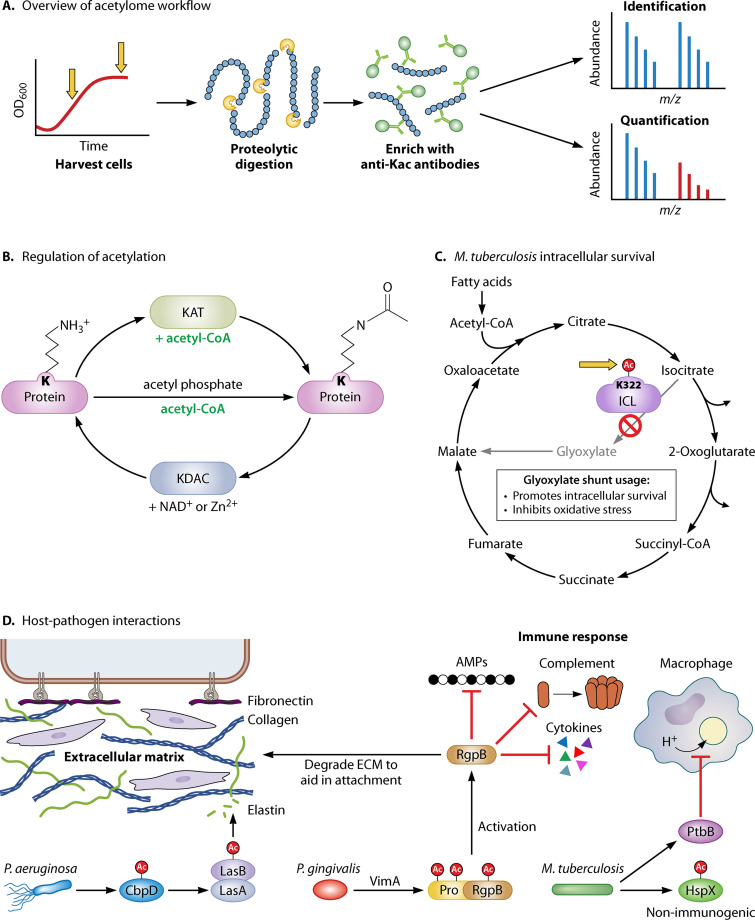
FIG 1.
(A) Typical acetylome workflow. Cells are harvested at different growth phases or under different conditions, and proteins are digested, typically with trypsin. Often, acetylated peptides are enriched using anti-acetyllysine antibodies conjugated to agarose beads. The acetylated peptides are identified and quantified by MS. OD600, optical density at 600 nm. (B) Summary of the regulation of acetylation in bacteria. Proteins are acetylated either enzymatically by lysine acetyltransferases (KATs) or nonenzymatically via the high-energy intermediate acetyl-CoA or acetyl-phosphate. Deacetylation occurs by the action of NAD+-dependent sirtuins or Zn2+-dependent lysine deacetylases (KDACs). (C) For intracellular pathogens such as M. tuberculosis, intracellular metabolism is altered by acetylating key enzymes to control usage of the glyoxylate shunt. The glyoxylate shunt avoids the CO2-producing steps of the TCA cycle and replenishes intermediates. Acetylation of isocitrate lyase (ICL) at K322 inhibits its enzymatic activity and likely blocks usage of the shunt. Thus, deacetylation of ICL may be a critical step for intracellular survival. (D) Many secreted virulence factors are acetylated, which suggests that acetylation mediates pathogen-host interactions. P. aeruginosa secretes the acetylated proteins CbpD and LasB, which lead to the degradation of the human extracellular matrix (ECM) component elastin and aid in tissue invasion. In P. gingivalis, acetylation of the inactive pro-RgpB is required for enzyme activation as a protease. The acetylated pro-RgpB is secreted, where it is activated and degrades ECM components and immune system components, including cytokines, antimicrobial peptides (AMPs), and complement proteins. In M. tuberculosis, heat shock protein X is secreted and, when acetylated, is nonimmunogenic. The activity of the secreted protein tyrosine phosphatase PtpB is controlled by acetylation. PtpB promotes intracellular survival by inhibiting acidification inside the phagolysosome.