FIGURE 3.
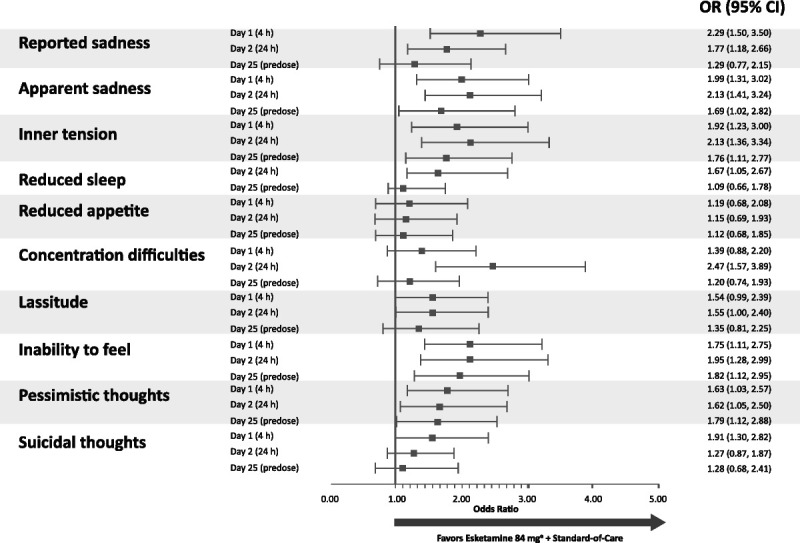
Odds ratios (95% CI) for a ≥2-point improvement in the MADRS item scores during the double-blind treatment phase. a Includes patients who had their dose reduced because of tolerability issues. Notes: MADRS items are rated on a scale of 0 to 6 where 0 indicates no abnormality and 6 indicates severe. The reduced sleep item was not assessed at the 4-hour post–first dose time point.
