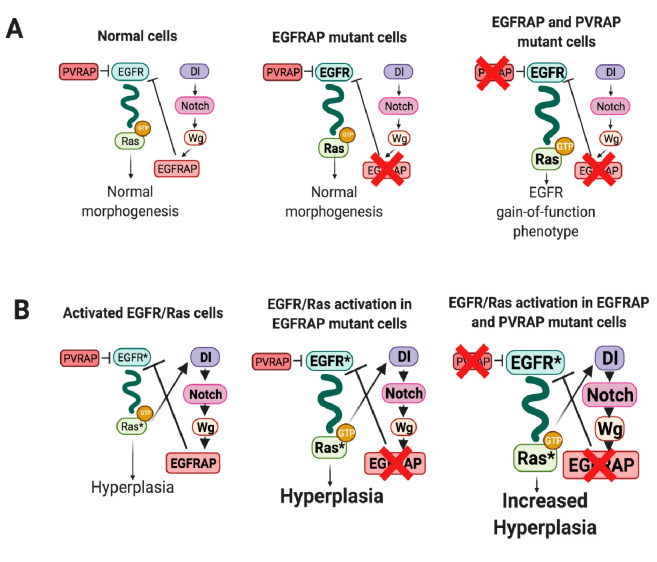
Fig 8. Model of EGFRAP function as a modulator of EGFR/Ras-dependent tissue hyperplasia.
Schematic drawing depicting the mechanisms by which EGFRAP could limit EGFR/Ras activity in normal (A) and RasV12-dependent oncogenic cells (B). (A) In normal cells EGFRAP and PVRAP act as negative regulator of the EGFR. Expression of EGRAP is confined to cells with high levels of EGFR activity via the Notch pathway. EGFRAP elimination results in a slight increase in EGFR activity, which does not seem to affect normal morphogenesis. However, the simultaneous elimination of EGFRAP and PVRAP leads to a further enhancement of EGFR signaling with consequences in normal morphogenesis. (B) Oncogenic EGFR/Ras activity promotes, via activation of the Notch pathway, an increase in EGFRAP expression that, in turn, restrains both EGFR activity and its capacity to induce hyperplasia. Downregulation of EGFRAP releases this restraint leading to a further increase in EGFR/Ras pathway activity and tumor growth, which is enhanced by elimination of PVRAP.