FIG 4.
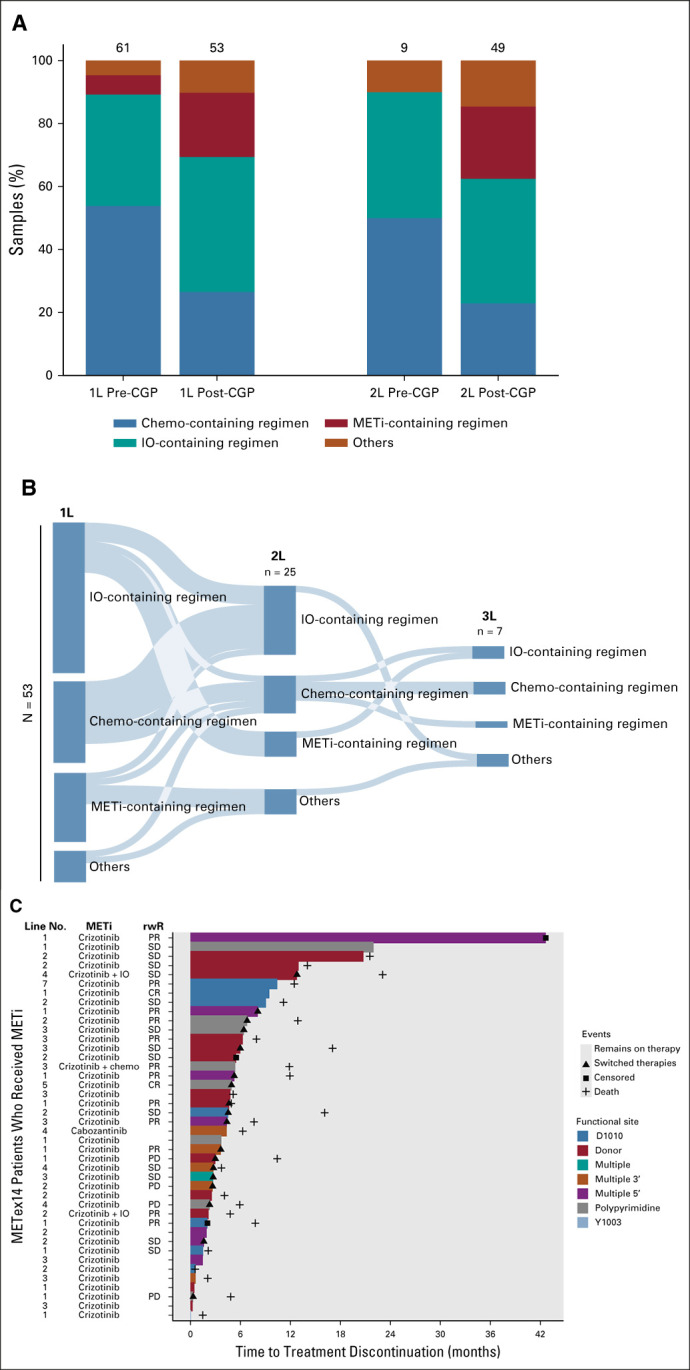
Real-world treatment patterns of patients with METex14-altered NSCLC in the CGDB. (A) Distribution of 1L and 2L therapies before and after CGP for 114 METex14-altered NSCLC who started treatment after CGP biopsy. All patients captured started 1L therapy between 2012 and 2020. (B) Sankey diagram of 53 patients with METex14-altered NSCLC with documented receipt of 1L therapy in the CGDB after report of CGP results. All patients captured started 1L therapy post-CGP report between 2015 and 2020. Categorical groupings include (1) METi-containing regimen: MET TKI monotherapy, MET TKI + chemotherapy, and MET TKI + IO, (2) IO-containing regimen: IO monotherapy, IO + chemotherapy, IO + clinical study drug, or IO + other targeted therapy, (3) chemo-containing regimen: chemotherapy monotherapy, chemotherapy + clinical study drug, or chemotherapy + other targeted therapy, and (4) other: non-MET targeted therapy (erlotinib, aftatinib, ceretinib, or bevacizumab) or unspecified clinical study drug, which may have included a MET TKI. Six patients who received MET TKI in the fourth to seventh lines are not captured here but are shown in (C). (C) Swimmer plot displaying time to treatment discontinuation for patients with METex14-altered NSCLC who received MET TKI. rwR was available for a subset of patients. 1L, first line; 2L, second line; CGP, comprehensive genomic profiling; CR, complete response; IO, immunotherapy; NSCLC, non–small-cell lung cancer; METi, MET inhibitors; PD, progressive disease; PR, partial response; rwR, real-world response; SD, stable disease; TKI, tyrosine kinase inhibitor.
