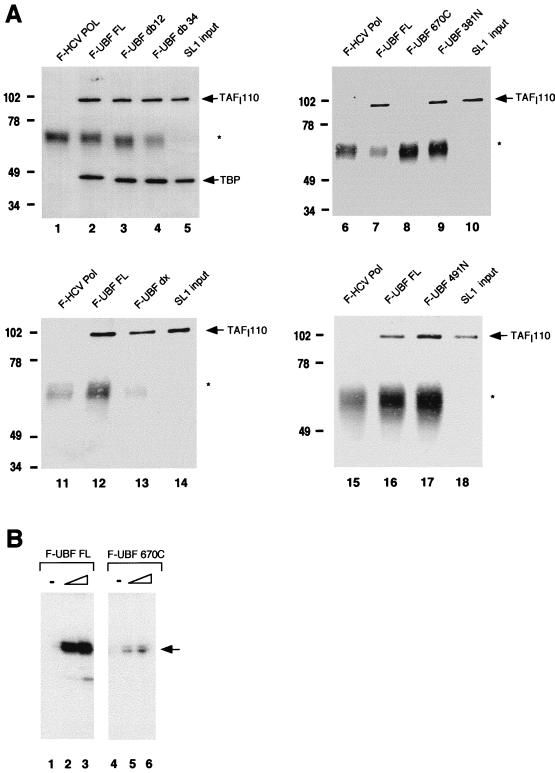
FIG. 2.
SL1 interacts with the carboxy-terminal domain of UBF. (A) Recombinant flag-tagged proteins were immobilized on anti-Flag M2 resin as bait and incubated with human SL1. The resulting complex was eluted, resolved on SDS-PAGE, and transferred to nitrocellulose for Western analysis to detect coimmunoprecipitated SL1 (see Materials and Methods). The bait proteins used in each reaction are as indicated above each panel. F-HCV Pol is the negative control, and the input is 10% of the SL1 used per reaction. Nitrocellulose was probed with polyclonal anti-TAFI110 antibody and reprobed with polyclonal anti-TBP antibody (lanes 1 to 5) or probed with anti-TAFI110 antibody alone (lanes 6 to 18). The asterisks denote immunoglobulin G heavy chain. Markers to the left of each gel show molecular mass in kilodaltons. (B) Increasing amounts (1 and 2 ng) of affinity-purified recombinant UBF FL (lanes 2 and 3) and UBF 670C (lanes 5 and 6) were used with partially purified RNA Pol I (2 μl; 5 mg/ml) and SL1 (1 μl; 0.8 mg/ml) in reconstituted transcription reactions. UBF amounts were estimated by SDS-PAGE and silver staining. In vitro-synthesized transcripts were detected by S1 nuclease protection assay. The arrow indicates the protected oligonucleotide fragment.