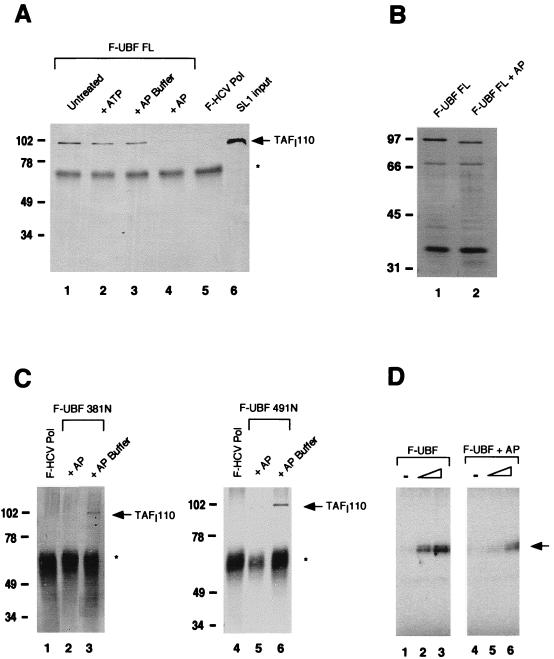
FIG. 3.
Role of UBF phosphorylation in SL1 binding. (A) F-UBF FL was immobilized on flag antibody beads and either untreated (lane 1) or treated with 5 mM ATP (lane 2), AP buffer alone (lane 3), or with buffer plus AP (lane 4). The binding assay was then performed as previously described. Coimmunoprecipitated SL1 was detected by Western blot analysis with anti-TAFI110 antibody. Lanes 5 and 6 are negative control and SL1 input, respectively. (B) Silver-stained SDS-PAGE of untreated UBF (lane 1) and AP-treated UBF (lane 2) after immunoprecipitation shows faster migration of dephosphorylated UBF. (C) F-UBF 381N and F-UBF 491N were immobilized on flag antibody beads and treated either with AP buffer alone (lanes 3 and 6) or with buffer plus AP (lanes 2 and 5). SL1 binding assays were done as previously described. (D) In vitro transcription assays containing partially purified RNA Pol I (10 μg), SL1 (0.8 μg), and increasing amounts (0.25 and 1.25 ng) of purified recombinant UBF (lanes 2 and 3) or dephosphorylated UBF (lanes 5 and 6) were performed as described in Materials and Methods. The transcription assays with UBF and AP-treated UBF were quantified with a phosphorimager. The mean fold activation in the presence of UBF or AP-treated UBF, calculated from two independent experiments, is 8.5- and 2.0-fold, respectively. Asterisks in panels A and C indicate immunoglobulin G heavy chain. Markers in panels A to C show molecular mass in kilodaltons. The arrow in panel D indicates the protected oligonucleotide fragment.