Abstract
Metaplastic breast carcinoma (MBC) is a rare malignant breast tumor, and no effective chemotherapy unique to metaplastic carcinoma exists. As MBC is typically “triple negative”, endocrine therapy and molecular therapy targeted to Her2 might not be favorable, resulting in a poor prognosis. Anlotinib is currently being tested in patients with breast or cancer. Here, we report a successful case in which anlotinib was used to treat MBC. A 54-year-old female patient visited the hospital after the discovery of a left breast tumor 10 months prior, and tumor redness and swelling had lasted for more than one month. After admission, relevant examinations were performed. After left breast tumor puncture revealed left emulsified biological cancer, the tumor significantly increased in size, and bleeding was obvious after 2 cycles of the “EC” chemotherapy regimen. The curative effect was evaluated as progressive disease (PD). After two cycles of chemotherapy with the “PCb” regimen, the efficacy was still PD. The Karnofsky performance status (KPS) score of the patient after 4 cycles of chemotherapy was 60 points, with severe anemia, and she could not tolerate chemotherapy. The patient was given radiotherapy to stop bleeding, and the tumor further increased in size during radiotherapy. The curative effect was evaluated as PD. After a multidisciplinary consultation in our hospital, we initiated oral anlotinib (12 mg; 2 weeks on, 1 week off). The tumor significantly decreased in size after taking anlotinib, and the efficacy was evaluated as PR. Adverse reactions during treatment were controlled, and progression-free survival (PFS) reached up to 25+ months. The follow-up is ongoing. The patient has provided written informed consent for the case details and images to be published, and at the same time institutional approval was required to publish the case details, we report this case.
Keywords: breast metaplastic carcinoma, anlotinib, targeted therapy
Introduction
Metaplastic breast carcinoma (MBC) is a rare malignant breast tumor with more than two kinds of tissue structures and an incidence of less than 1%.1 MBC usually does not express estrogen receptor (ER), progesterone receptor (PR) and Cerb-B2 (ie, triple-negative breast cancer). As for triple-negative breast cancer, surgery and chemotherapy are the main treatments for early patients. Chemotherapy is still the most common primary treatment for advanced triple-negative breast cancer. Immunotherapy has entered the clinical practice of triple-negative breast cancer, and PD-L1 positive population is the preferred population for first-line immunotherapy. Some targeted therapies have made precise breakthroughs in a subset of patients with advanced triple-negative breast cancer, and ADC drugs have promising application prospects in advanced triple-negative breast cancer. But differing from ordinary triple-negative breast cancers. MBC is not sensitive to chemotherapy, and the main treatment is surgery,2 so it is crucial to search for new treatments for metaplastic breast carcinoma.
Anlotinib is a multitarget receptor tyrosine kinase inhibitor originally developed in China. It can inhibit the multiple kinase activities of VEGFR1, VEGFR2, VEGFR3, c-Kit, and PDGFRβ, has a strong inhibitory effect on angiogenesis pathways and can inhibit the proliferation and migration of tumor cells.3 Angiogenesis is a key mechanism of tumor growth. The occurrence, development and metastasis of malignant tumors are closely related to angiogenesis. Anlotinib has achieved good curative effect in the treatment of non-small cell lung cancer (NSCLC), small cell lung cancer (SCLC), soft tissue sarcoma, esophageal cancer and other fields.4–7 However, in the treatment of breast cancer, the use of anlotinib has not been reported much. This case report describes a patient with breast cancer who still progressed rapidly after second-line chemotherapy. After a multidisciplinary consultation, the oral administration of anlotinib achieved good efficacy and good tolerance. This suggests that anlotinib can be used as a treatment for advanced or locally advanced metaplastic breast cancer.
Case Description
Patient Concerns
In October 2018, a 54-year-old female patient visited the hospital because a left breast tumor was discovered 10 months prior, and tumor redness and swelling had lasted for more than one month. At the beginning of 2018, the patient discovered a left breast tumor resembling peanut rice without pain and nipple discharge, erosion or other symptoms. There was no special diagnosis or treatment. In September 2018, the patient’s tumor increased significantly in size, accompanied by redness, swelling and bloating pain, and the pain was not related to the menstrual cycle. Subsequently, the tumor broke, and a light red and light yellow liquid outflow was observed. She was sent to our hospital for further diagnosis and treatment.
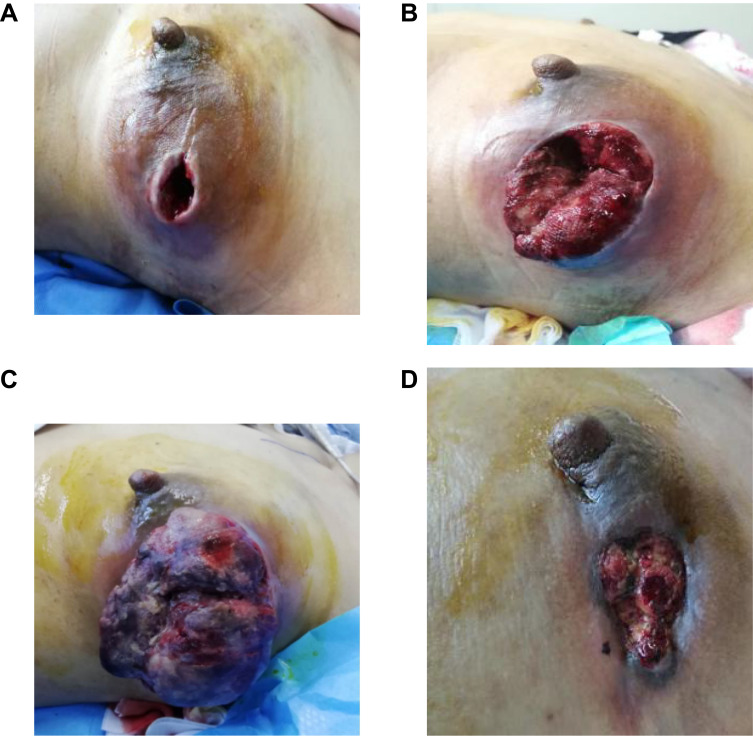
Physical examination on admission (Figure 1A): The appearance of the breasts was asymmetrical, and the left breast was red and swollen and larger than the right breast. There was a defect in the upper outer quadrant of the left breast, and the gap was approximately 5*4*5 cm. A palpable mass measuring approximately 15*10*8 cm, hard and fixed on the chest wall, was observed in the left breast central region and external quadrant. The right breast was not abnormal, and the bilateral axillary and supraclavicular lymph nodes were not palpable. Auxiliary examination: left breast dressing covered, failed to photograph. Breast ultrasound showed the following: 1, left breast dressing coverage failed to film, right breast no obvious occupation; and 2, left subclavian to left axillary abnormal echo, size 26*11 mm, considering lymph node enlargement, metastasis to be rowed (metastatic?). Mammography, CT, and SPECT showed no abnormalities. As the patient was noncooperative, MRI of the breasts could not be performed.
Figure 1.
The changes of Physical examination. (A) Physical examination on admission. (B) “EC” chemotherapy. (C) “PCb” chemotherapy. (D) the administration of anlotinib.
Diagnoses
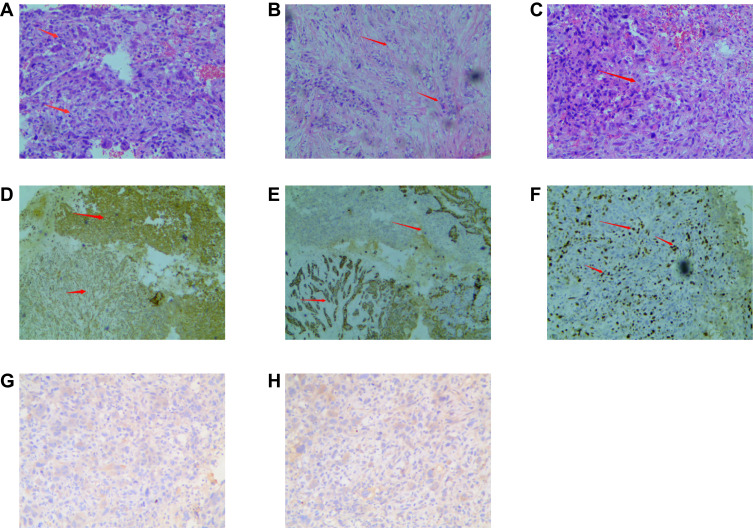
After completing the relevant examinations and considering the taboo associated with invasive operations, the patient underwent a “left breast tumor puncture”. The postoperative pathological examination (Figure 2A–H) showed some lamellar tumor cells, different sized cells, atypia, coarse chromatin, obvious nuclear division, and osteoclast-like cells. Some tumor cells were tubular, while some were fusiform and atypical, with different sizes and in different stages of nuclear division. Cancer and sarcoma components, mostly sarcoma components, overlap and are difficult to distinguish. Necrosis was observed in the center of the tumor. Immunohistochemistry: ER(-), PR(-), HER2(0), Ki-67(40%), Vim(+), GCDFP-15(+), MC(-), P63(+), CK5/6(+), CK8(+), and P53(+). Pathological diagnosis (left breast mass puncture): conforms to metaplastic carcinoma with osteoclast-like giant cells, mostly with sarcoma components, including invasive ductal carcinoma and spindle cell carcinoma. The postoperative pathological examination of the “left axillary and subclavian lymph node puncture” showed inflammation with severe atypical hyperplasia. Based on the pathological results, the mass was clearly diagnosed as left emulsification bioplastic carcinoma, stage cT4N3M0, stage IIIC. Neoadjuvant therapy for breast cancer was proposed.
Figure 2.
The pathological examination of postoperative. (A) Some lamellar tumor cells, different sized cells, atypia, coarse chromatin, obvious nuclear division, and osteoclast-like cells. (B) Some tumor cells were tubular, while some were fusiform and atypical, with different sizes and in different stages of nuclear division. (C) Some cells are necrosis in the center of the tumor. (D) VIM (+) (4X10). (E) CD56 (+) (4X10). (F) ki-67 40% (4X10). (G) ER (-) (10X10). (H) PR (-) (10X10).
Interventions
On October 20, 2018, the patient was given the “EC” chemotherapy regimen (epirubicin 130 mg d1; cyclophosphamide 0.8 mg d1), and its efficacy was evaluated as progressive disease (PD) after 2 cycles (Figure 1B). Considering that the patient had left emulsified metaplastic carcinoma, the molecular classification was triple negative, and the treatment plan was changed to the “PCb” chemotherapy regimen (albumin paclitaxel 150 mg d1/8/15; carboplatin 0.2 g d1/8/15) for 2 cycles. During chemotherapy, the patient’s mass increased significantly (Figure 1C), bleeding was obvious, and the efficacy was still evaluated as PD. At this time, the patient’s physical condition had deteriorated, with a Karnofsky performance status (KPS) score of 60 and severe anemia. Considering that the patient could not tolerate chemotherapy, PARP inhibitor therapy was proposed, but no pathogenic BRCA1/2 gene exon mutation was detected. After communicating with a radiotherapist, palliative radiotherapy was recommended for the patient to stop bleeding. Intensity-modulated conformal radiotherapy (IMRT) was performed, and the total radiation dose and segmentation method were as follows: planning target volume (PTV) DT45 Gy/15 F/21 d for and DT300 cGy/F for chest bone metastases. After 2 rounds of radiotherapy, the left breast tumor spontaneously detached during the dressing change. The exfoliated tissue was sent for a pathological examination. Microscopically, necrotic tissue with inflammatory cell infiltration, local abscess formation, and a small amount of cancer tissue were observed. Immunohistochemistry: ER (-), PR (-), HER-2 (0), and Ki-67 (+, 30%). However, during subsequent radiotherapy, the left breast tumor further increased in size, and at the same time, a mass of approximately 4*10*3 cm in size and soft with a sense of fluctuation was palpated at the sternal stalk. Re-examination of the ultrasound findings showed a mixed cyst-solid space in the subcutaneous soft tissue of the sternum (range: approximately 86*32*27 mm), and metastases were possible. CT findings: sternal destruction with a soft tissue mass, metastasis considered. PET/CT findings: sternal bone destruction with metabolic abnormalities, metastasis considered. PET/CT imaging of other parts of the body showed no obvious abnormalities. At this time, the patient was diagnosed with left breast metaplasia, stage cT4N3M1, stage IV, and left breast cancer with sternal metastasis. After communication, a tissue-based NGS assay was performed, showing a MAP2K1 deletion mutation, along with TP53 p.S183X on exon 5 and TP53 p. S241F on exon 7 mutations, with a low tumor mutational burden (TMB), and microsatellite stability. Drug sensitivity test results showed that the drugs with high sensitivity were carboplatin, taxon, cyclophosphamide, letrozole, and mitomycin. After consultation with the multidisciplinary team (MDT) in our hospital, the patient was diagnosed with left emulsification metaplasia, stage cT4N3M1, stage IV, left breast cancer with sternal metastasis. “Replace chemotherapeutics±antiangiogenic drugs” was recommended. Considering that the patient’s physical condition was still poor, with a KPS score of 70, after communicating with the patient and her family, they indicated that chemotherapy was not an option, and radiotherapy was voluntarily suspended. Finally, a single drug, “anlotinib” (12 mg orally) was administered for two weeks and stopped for one week.
Outcomes
After the administration of anlotinib, the tumor spontaneously detached again and did not increase in size (Figure 1D), and the sternum metastasis was stable. After 2 weeks, the blood pressure of the patients was high (up to 140/90 mmHg). After treatment in the Department of Cardiology, the patient was given oral “nifedipine controlled-release tablets”, and her blood pressure was maintained at 110–120/70–90 mmHg without adjusting the dose of anlotinib. Currently, the patient continues to take anlotinib, the progression-free survival (PFS) duration is up to 25 months, the efficacy is evaluated as PR, the drug is well tolerated, and the patient continues follow-up.
Discussion
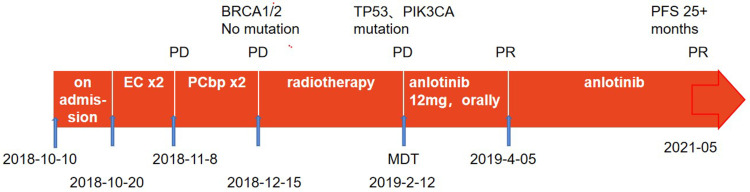
In this case, a 54-year-old female patient was diagnosed with MBC after 2 cycles of the “EC” chemotherapy regimen, and the efficacy was evaluated as PD. According to RECIST 1.1 criteria for tumor evaluation, PD (Progressive Disease) means disease progression, the minimum sum of the diameter of all target lesions measured during the whole experimental study was used as reference, and the relative increase in diameter and relative increase of at least 20% (if the baseline measurement was minimum, the baseline value was used as reference); In addition, an absolute increase of at least 5 mm in the diameter sum must be satisfied (the presence of one or more new lesions is also considered disease progression). The patient’s tumor showed visible growth, which was much larger than the value of therapeutic efficacy assessment PD, so the therapeutic efficacy assessment was PD. The efficacy of the “PCb” chemotherapy regimen after 2 cycles remains PD. The KPS score of the patient after 4 cycles of chemotherapy was 60 points, with severe anemia, and chemotherapy could not be tolerated. The patient was treated with radiotherapy to stop bleeding, but the tumor further increased in size during radiotherapy. The curative effect was evaluated as PD. After consultation with the multidisciplinary team in our hospital, oral anlotinib was started. After taking anlotinib, the tumor size decreased significantly, and the efficacy was evaluated as PR. Adverse reactions during treatment were controllable and safe. To date, the patient has been receiving oral anlotinib, with a PFS duration of 25 + months. In summary, it is my opinion that this complete treatment process was very successful (Figure 3).
Figure 3.
Complete treatment process.
The pathological examination of the left breast tumor after puncture in this case showed metaplastic carcinoma with high-grade sarcoma components and triple-negative immunohistochemical results. The age of onset, postoperative pathological manifestations and immunohistochemical classification of the patient were consistent with the literature.8,9 Metaplastic breast cancer is a rare malignant breast tumor.1 Relevant data show that its age of onset is between 48 and 61.1 years.8 Histologically, it is usually composed of malignant epithelial components and interstitial components, so most manifest as both cancer and sarcoma.9 In 2012, according to its morphological characteristics, the WHO divided MBC into five subtypes: low-grade adenosquamous carcinoma, fibromatoid carcinoma, mesophyll differentiated carcinoma, squamous cell carcinoma and spindle cell carcinoma.10
Immunohistochemically, more than 90% of breast metaplastic carcinomas are triple-negative, basal-like carcinomas. Therefore, the chemosensitivity of this cancer to endocrine therapy and anti-HER2 targeted therapy has been determined.11 Due to the lack of treatment recommendations for metaplastic cancers, the treatment is still based mainly on invasive breast cancer, and chemotherapy options remain dominated by taxanes and anthracyclines.12
The patient in this case received 2 cycles of EC and 2 cycles of PCb chemotherapy after a confirmed diagnosis. During chemotherapy, the tumor progressed rapidly, showing insensitivity to chemotherapy drugs, which was consistent with the results reported in the relevant literature.2 At present, the patient is faced with tumor hemorrhage and a poor physical status. As a result, the patient has been given symptomatic and supportive treatment combined with radiotherapy for local hemostasis. However, it is difficult to further shrink the tumor with radiotherapy.
In 2020, Professor Shao Zhimin’s FUTURE study13 first proposed the “Fudan classification” for triple-negative breast cancer, which was divided into four different subtypes: immunomodulatory (IM), luminal androgen receptor (LAR), basal-like immunosuppressive (BLIS) and mesenchymal (MES). Accurate treatment was performed according to the different subtypes, and the objective remission rate (ORR) of triple-negative breast cancer patients with multiline resistance at or above the fourth line reached 29%. The subgroup “basal-like immunosuppressive subtype BRCA mutation group” was treated with the “PARP inhibitor fluzoparib” regimen. Although the ORR and disease control rate (DCR) were 0%, the results inspired us to try an alternate therapy. Considering that in this case, MBC was the basal-like type, the BRCA1/2 gene test was performed, but the results indicated no pathogenic mutation. In the FUTURE study, patients in the “basal-like immunosuppressive subtype BRCA nonmutant group” were treated with the “VEGFR inhibitor apatinib” regimen, and the ORR reached 26%. However, different from the FUTURE study, the Eastern Cooperative Oncology Group (ECOG) score of the patient in this case was only 3 points, and this patient had a large breast tumor burden and was bleeding continuously. After radiotherapy, the tumor spontaneously detached, but the breast tumor still further increased in size during subsequent radiotherapy.
Subsequently, a blood-based next-generation sequencing (NGS) assay was performed, showing a TP53 p.G245C on exon 7 and PIK3CA p.H1047R on exon 20 mutations. In the field of gene detection, with the rapid development of high-throughput gene analysis technology, breast multigene expression profiles have been gradually applied in clinical practice, and the research direction is gradually developing up to the molecular level. Mutations in genes such as TP53 and PIK3CA and deletions of PTEN and kinase inhibitor 2A have been gradually found in cancerous tissues. The mutation rate of the TP53 gene is as high as 50% in human tumors, 20–40% in breast cancer, and 61% in metaplastic breast cancer.14 Tp53 is a tumor suppressor protein encoded by the TP53 gene, which is located at 17p13. 1. It regulates the cell cycle at G1/S and initiates apoptosis. Moreover, 71% Tp53 overexpression was found in 14 cases of breast metaplastic carcinoma.15 There is no targeted treatment for tumors with a TP53 gene mutation. However, since TP53 gene mutations can affect other tumor growth pathways (eg, by increasing TP53 gene and VEGF levels), tyrosine kinase inhibitors may inhibit tumor growth and progression by effectively inhibiting tumor angiogenesis in MBC.16 Said et al17 showed that the median PFS (mPFS) of patients with TP53 gene mutations who received the antiangiogenic drug bevacizumab was 11 months (95% CI: 5.9–16.0) and that of patients who did not receive bevacizumab was only 4 months (95% CI: 3.6–5.7) (p < 0.001). Recently, Onco Targets and Therapy reported three advanced lung cancer patients with TP53 mutations, which responded well to anlotinib.18 Other authors also reported the role of the Tp53 protein in inhibiting angiogenesis and angiogenesis.19,20 This is consistent with some previous reports that TP53 mutations are sensitive to angiogenesis, suggesting that patients with Tp53 mutations may be a potential benefit group for anlotinib.
Some patients also have PIK3CA gene mutations, and the incidence of PIK3CA gene mutations in breast cancer is approximately 8%–40%.21 Some researchers have suggested that the PI3K/mTOR/Akt signaling pathway may also be a potential therapeutic target for MBC. The PI3K signaling pathway can affect cell growth, survival and metabolism. In tumor cells, the PI3K signaling pathway is activated by a direct mutation or amplification of a gene encoding the key components of the PI3K signaling pathway (such as PIK3CA and AKT1); alternatively, the deletion of tumor suppressor phosphatase and angiotensin homolog (PTEN) on chromosome 10 leads to the activation of the PI3K signaling pathway. Inhibition of the PI3K signaling pathway can reduce cell proliferation and promote cell death and may affect tumor angiogenesis and metastasis. At present, many PI3K inhibitors have been approved for marketing or entered clinical studies, such as Buparlisib, Pictilisib (GFC-0941), Copanlisib, and so on, but their progress is slow due to severe side effects and toxicity. Some selective PI3K inhibitors have been approved, such as Alpelisib, the first FDA-approved PI3K inhibitor for the treatment of breast cancer. Currently, PI3K inhibitors have been approved mainly for breast cancer patients with HR+, and the unknown effect of TNBC is a new area worth exploring.
The three signaling pathways mediated by VEGFR, PDGFR and FGFR and their interactions are the main mechanisms through which tumor angiogenesis is regulated. Among them, the signaling pathway mediated by VEGFR-2 is the most important. Therefore, VEGFR2 is the main target for current vascular-targeting drugs. It is a pity that VEGFR2 changes were not detected after treatment due to the patient’s long journey and the high cost of genetic test in this case.
Bevacizumab is a recombinant human monoclonal IgG1 antibody that mainly inhibits tumor angiogenesis by blocking the binding of VEGF-A and VEGFR-2. Apatinib is a small-molecule tyrosine kinase inhibitor that targets vascular endothelial growth factor receptor-2 (VEGFR-2), which can strongly inhibit tumor angiogenesis and thus exert antitumor effects. Anlotinib is a multitarget receptor tyrosine kinase inhibitor that can inhibit the activities of VEGFR- 1, VEGFR-2, VEGFR-3, c-Kit, PDGFRβ and other kinases. It has a strong inhibitory effect on the angiogenesis pathway and inhibits the proliferation and migration of tumor cells.
Combining the FUTURE study and the results of the patient’s genetic test results, the patient consulted a multidisciplinary team. The team recommend “replacement of chemotherapeutic drugs ± antiangiogenic drugs”, and antiangiogenic treatment was planned after a discussion with the patient and her family. In clinical practice, bevacizumab generally needs to be combined with chemotherapy, but the patient described herein could not tolerate chemotherapy, so it was not considered. Apatinib has relatively large adverse reactions and may have a higher bleeding tendency than anlotinib8,22. In this patient, the tumor was bleeding, so the use of apatinib was not considered. Finally, after a discussion, the antiangiogenetic drug anlotinib was chosen. Previously, anlotinib showed a significant effect in the treatment of sarcoma.6,7
A basic study published in Cell Death and Disease in 202023 evaluated the antitumor effect of anlotinib in a xenograft mouse model of intrahepatic cholangiocarcinoma. In two types of intrahepatic cholangiocarcinoma (ICC) cells, anlotinib reversed the phosphorylation of VEGFR2 and AKT induced by VEGF, thereby regulating the expression of related proteins in the downstream signaling pathway, such as the upregulation of E-cadherin and Bax and the downregulation of N-cadherin, Bcl-2, survivin and CDK1. In addition, knocking down VEGFR2 reversed the effect of anlotinib. Compared with the control group in the patient-derived xenograft (PDX) tumor model, the phosphorylation levels of VEGFR2 and AKT and the protein expression level of CDK1 in the anlotinib group were significantly reduced. This study has proven that anlotinib has excellent antitumor activity in ICC, mainly by inhibiting the phosphorylation of VEGFR2 and AKT, thereby inhibiting downstream PI3K/AKT signal transduction, inhibiting the proliferation and invasion of tumor cells, and promoting apoptosis. Another basic study24 showed that anlotinib could block the phosphorylation of AKT, inhibit the PI3K/AKT signaling pathway, and inhibit the expression of PD-L1 on human umbilical vein endothelial cells (HUVECs). This study confirmed that through the inhibition of the PI3K/AKT signaling pathway combined with changes in CD8+ T cells and other mechanisms, anlotinib improved the tumor immune microenvironment and provided a theoretical and experimental basis for the clinical application of anlotinib combined with immunotherapy. As an exploratory treatment, if disease control is not satisfactory during the use of anlotinib, combined immunotherapy can be considered, and the safety and tolerability of drug combinations should be a focus.
Moulder et al25 found that mTOR inhibitors combined with antiangiogenic drugs could further inhibit tumor growth. Fifty-two female MBC patients in the Phase I trial were treated with liposomal doxorubicin combined with bevacizumab and temsirolimus or everolimus, and the objective response rate reached up to 21%. At the same time, researchers found that an abnormal PI3K pathway was closely related to a significant increase in the objective response rate in these patients (31% vs 0%, P=0.04). Unfortunately, PI3K and mTOR inhibitors are not currently approved in China. However, the CCN protein family and PARP inhibitors also provide a new direction for targeted therapy in MBC.
Previously, anlotinib showed a significant effect in the treatment of sarcoma.4–7 Some MBCs derived from mesenchymal tissue show sarcoma components. The pathological results of this patient showed breast metaplastic carcinoma with high-grade sarcoma components. Anlotinib treatment maintained long-term PFS, which may be related to the sarcoma components. In recent years, anlotinib has emerged in the treatment of metastatic breast cancer. In 2021, Professor Yuan Peng’s team from the Cancer Hospital of the Chinese Academy of Medical Sciences published a study on the treatment of HER-2-negative metastatic breast cancer with anlotinib.26 The ORR was 15%, the mPFS was 5.22 months, the DCR was 80.77%, and the safety was good. The grade ≥3 treatment-related adverse events of were hypertension (26.92%) and hand-foot syndrome (3.85%), and there were no treatment-related deaths.
Finally, the patient with metaplastic cancer in this case is currently being treated with anlotinib to have a better effect. After symptomatic treatment, the adverse reactions were controlled. Metaplastic carcinoma is a rare cancer with a poor prognosis.12 Whether anlotinib provides a new treatment option for these patients and whether anlotinib has a good therapeutic effect in breast cancer patients with TP53 and PIK3CA gene mutations remain unclear. These issues need to be further investigated.
Acknowledgments
The authors thank the patient for her agreement with the publication of this report.
Abbreviations
MBC, metaplastic breast carcinoma; EC, epirubicin and cyclophosphamide; PD, disease progression; PCb, abraxane and carboplatin; KPS, Karnofsky performance status; PFS, progression-free survival; mPFS, median progression-free survival; MDT, multidisciplinary team consultation; ER, express estrogen receptor; PR, progesterone receptor; PR, partial response; NSCLC, non-small cell lung cancer; SCLC, small cell lung cancer; IMRT, intensity-modulated conformal radiotherapy; PTV, planning target volume; TMB, tumor mutational burden; IM, immunomodulatory; LAR, luminal androgen receptor; BLIS, basal-like immunosuppressive; MES, mesenchymal; ORR, objective remission rate; DCR, disease control rate; ECOG, the Eastern Cooperative Oncology Group; ICC, intrahepatic cholangiocarcinoma; VEGFR, inhibit vascular endothelial growth factor receptor; PDGFR, platelet-derived growth factor receptor; FGFR, fibroblast growth factor receptor; TKI, tyrosine kinase inhibit; PDX, patient-derived xenograft; HUVECs, human umbilical vein endothelial cells; IHC, immunohistochemistry; RT-PCR, reverse transcription-polymerase chain reaction.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Luini A, Aguilar M, Gatti G, et al. Metaplastic carcinoma of the breast, an unusual disease with worse prognosis: the experience of the European Institute of Oncology and review of the literature. Breast Cancer Res Treat. 2007;101:349–353. doi: 10.1007/s10549-006-9301-1 [DOI] [PubMed] [Google Scholar]
- 2.Lee H, Jung SY, Ro JY, et al. Metaplastic breast cancer: clinicopathological features and its prognosis. J Clin Pathol. 2012;65(5):441–446. doi: 10.1136/jclinpath-2011-200586 [DOI] [PubMed] [Google Scholar]
- 3.Syed YY. Anlotinib: first global approval. Drugs. 2018;78(10):1057–1062. doi: 10.1007/s40265-018-0939-x [DOI] [PubMed] [Google Scholar]
- 4.Cheng Y, Wang QM, Li K, et al. Anlotinib as third-line or further-line treatment in relapsed SCLC: a multicenter, randomized, double-blind Phase 2 trial. IASLC 19th World Conference on Lung Cancer. Toronto: IASLC; 2018. Abstract OA13.03. [Google Scholar]
- 5.Han B, Li K, Wang Q, et al. Third-line treatment: a randomized, double-blind, placebo-controlled Phase III ALTER-0303 study—efficacy and safety of anlotinib treatment in patients with refractory advanced NSCLC. J Clin Oncol. 2017;35:9053. doi: 10.1200/JCO.2017.35.15_suppl.9053 [DOI] [Google Scholar]
- 6.Chi Y, Sun Y, Cai J, et al. Phase II study of anlotinib for treatment of advanced soft tissues sarcomas. J Clin Oncol. 2016;34(15 Suppl):11005. doi: 10.1200/JCO.2016.34.15_suppl.11005 [DOI] [Google Scholar]
- 7.Chi Y, Yao Y, Wang S, et al. Anlotinib for metastasis soft tissue sarcoma: a randomized, double-blind, placebo-controlled and multi-centered clinical trial. J Clin Oncol. 2018;36(15 Suppl):11503. doi: 10.1200/JCO.2018.36.15_suppl.11503 [DOI] [Google Scholar]
- 8.Pezzi CM, Patel-Parekh L, Cole K, et al. Characteristics and treatment of metaplastic breast cancer: analysis of 892 cases from the National Cancer Data Base. Ann Surg Oncol. 2007;14:166–173. doi: 10.1245/s10434-006-9124-7 [DOI] [PubMed] [Google Scholar]
- 9.Hu Q, Chen WX, Zhong SL, et al. Current progress in the treatment of metaplastic breast carcinoma. Asian Pac J Cancer Prev. 2013;14(11):6221–6225. doi: 10.7314/APJCP.2013.14.11.6221 [DOI] [PubMed] [Google Scholar]
- 10.Lakhani SR, Ellise IO, Schnitt SJ, et al. WHO Classification of Tumours of the Breast. 4th ed. Lyon, France: IARC; 2012. [Google Scholar]
- 11.Wang H, B. G, Shi Q, et al. May metaplastic breast carcinomas be actually basal like carcinoma? Further evidence study with its ultrastructure and survival analysis. Med Oncol. 2011;28(1):42–50. doi: 10.1007/s12032-009-9399-1 [DOI] [PubMed] [Google Scholar]
- 12.Terando AM, Agnese DM, Holmes DR. Treatment and prognosis of rare breast cancers. Ann Surg Oncol. 2015;22(10):3225–3229. doi: 10.1245/s10434-015-4748-0 [DOI] [PubMed] [Google Scholar]
- 13.Jiang Y-Z, Liu Y, Xiao Y, et al. Molecular subtyping and genomic profiling expand precision medicine in refractory metastatic triple-negative breast cancer: the FUTURE trial. Cell Res. 2021;31:178–186. doi: 10.1038/s41422-020-0375-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borresen-Dale AL. TP53 and breast cancer. Hum Mutat. 2003;21:292–300. doi: 10.1002/humu.10174 [DOI] [PubMed] [Google Scholar]
- 15.Lien HC, Lin CW, Mao TL, et al. Tp53 overexpression and mutation in Metaplastic carcinoma of the breast: genetic evidence for a monoclonal origin of both the carcinomatous and the heterogeneous sarcomatous components. J Pathol. 2004;204:131–139. doi: 10.1002/path.1624 [DOI] [PubMed] [Google Scholar]
- 16.Bellino R, Arisio R, D’Addato F, et al. Metaplastic breast carcinoma: pathology and clinical outcome. Anticancer Res. 2003;23:669–673. [PubMed] [Google Scholar]
- 17.Said R, Hong DS, Warneke CL, et al. TP53 mutations in advanced cancers: clinical characteristics, outcomes, and correlation between progression-free survival and bevacizumab-containing therapy. Oncotarget. 2013;4:705–714. doi: 10.18632/oncotarget.974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fang S, Cheng W, Zhang M, et al. Association of TP53 mutations with response to anlotinib treatment in advanced non-small cell lung cancer. Onco Targets Ther. 2020;13:6645–6650. doi: 10.2147/OTT.S257052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koehler K, Liebner D, Chen JL. TP53 mutational status is predictive of pazopanib response in advanced sarcomas. Ann Oncol. 2016;27(3):539–543. doi: 10.1093/annonc/mdv598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu Y, Huang J, Wang Q, et al. Whole-exome sequencing insights into pulmonary artery sarcoma mimicking pulmonary embolism: a case report and review. Onco Targets Ther. 2019;12:6227–6235. doi: 10.2147/OTT.S212416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kataoka Y, Mukohara T, Shimada H, et al. Association between gain-of-function mutations in PIK3CA and resistance to HER2-targeted agents in HER2-amplified breast cancer cell lines. Ann Oncol. 2010;21(2):255–262. doi: 10.1093/annonc/mdp304 [DOI] [PubMed] [Google Scholar]
- 22.Das A, Mahapatra S, Bandyopadhyay D, et al. Bleeding with vascular endothelial growth factor tyrosine kinase inhibitor: a network meta-analysis. Crit Rev Oncol Hematol. 2021;57:103186. doi: 10.1016/j.critrevonc.2020.103186 [DOI] [PubMed] [Google Scholar]
- 23.Song F, Hu B, Cheng J-W. Anlotinib suppresses tumor progression via blocking the VEGFR2/PI3K/AKT cascade in intrahepatic cholangiocarcinoma. Cell Death Dis. 2020;11:573. doi: 10.1038/s41419-020-02749-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu S, Qin T, Liu Z, et al. Anlotinib alters tumor immune microenvironment by downregulating PD- L1 expression on vascular endothelial cells. Cell Death Dis. 2020;11(5):309. doi: 10.1038/s41419-020-2511-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moulder S, Moroney J, Helgasonn T, et al. Responses to liposomal doxorubicin, bevacizumab, and temsirolimus in metaplastic carcinoma of the breast: biologic rationale and implications for stem-cell research in breast cancer. J Clin Oncol. 2011;29(19):e572–e575. doi: 10.1200/JCO.2010.34.0604 [DOI] [PubMed] [Google Scholar]
- 26.Hu N, Si Y, Yue J, et al. Anlotinib has good efficacy and low toxicity: a phase II study of anlotinib in pre-treated HER2 negative metastatic breast cancer. Cancer Biol Med. 2021;18:2095–3941. doi: 10.20892/j.issn.2095-3941.2020.0463 [DOI] [PMC free article] [PubMed] [Google Scholar]