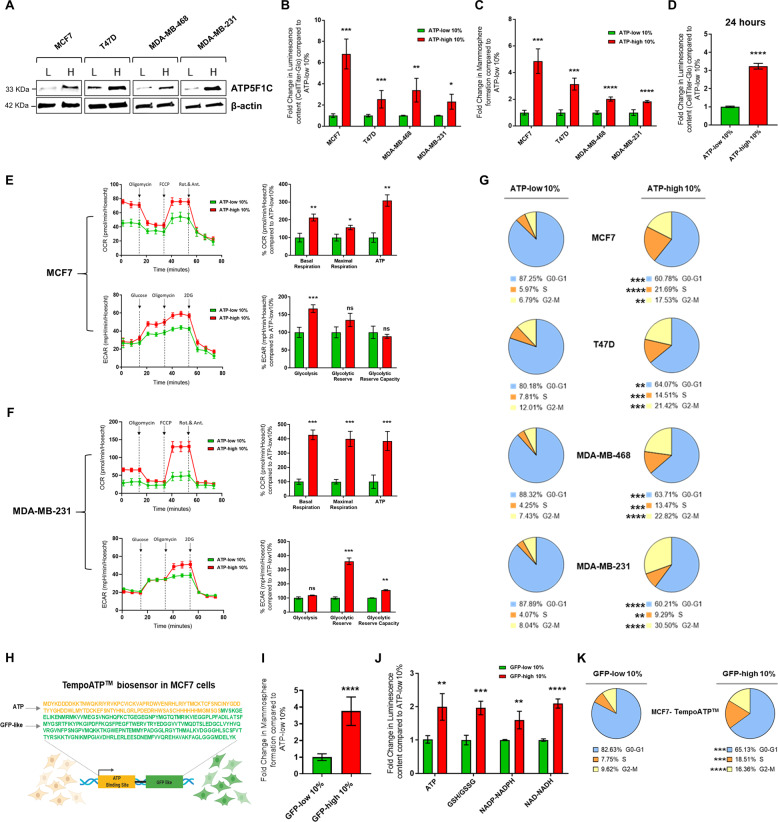
Fig. 3. ATP-high subpopulations of MCF7, T47D, MDA-MB-231, and MDA-MB-468 cells all overexpress ATP5F1C, are hyper-metabolic, and show increased 3D anchorage-independent growth, as well as elevated cell cycle progression.
A ATP5F1C is overexpressed in the ATP-high cell subpopulation. After metabolic fractionation, the ATP-high and ATP-low subpopulations were subjected to Western blot analysis with a mono-specific antibody probe that specifically recognizes ATP5F1C. Note that ATP5F1C is overexpressed in the ATP-high subpopulation in all four cell lines tested. B ATP levels after metabolic fractionation. The relative amount of ATP in the ATP-high and ATP-low cell subpopulations was independently validated using Cell-Titer-Glo. 2D monolayer cells were first stained with BioTracker ATP-Red 1 and sorted by ATP content, using a flow cytometer. After cell counting, equal numbers of single cells were then used to evaluate their relative luminescence content. In this series of experiments, we used a cut-off of 10% to define the ATP-high and ATP-low cell populations. Note that this metabolic fractionation scheme can be successfully applied to other breast cancer cell lines. Data represent the mean fold increase over ATP-low 10% cells ±SD, n = 3. Unpaired t test, *p < 0.05, **p < 0.005, ***p < 0.0005. C Mammosphere formation. Anchorage-independent growth was quantitated using the 3D mammosphere formation assay. In this series of experiments, we used a cut-off of 10% to define the ATP-high and ATP-low cell populations. Cells in 2D monolayers were first stained with BioTracker ATP-Red 1 and sorted by ATP content, using a flow cytometer. After cell counting, 5 × 103 cells were seeded onto a poly-HEMA coated 6-well plate and counted after 5 days. Note that the ATP-high cell population of MCF7, T47D, MDA-MB-231, and MDA-MB-468 cells all showed an increased capacity for 3D anchorage-independent growth. Data represent the mean fold increase over ATP-low 10% cells ±SD, n = 3. Unpaired t test, ***p < 0.0005, ****p < 0.0001. D ATP levels remain high, even 24 h after plating. ATP levels in different MCF7 cell subpopulations were quantitated using Cell-Titer-Glo analysis. After cell counting, equal numbers of single cells were then used to evaluate their relative ATP content by luminescence using the Varioskan™ LUX plate reader, 24 h after plating. For these experiments, which required larger numbers of cells, we used a cut-off of 10% to define the ATP-high and ATP-low cell populations. Note that ATP-high cells showed a greater than threefold increase in ATP levels, as compared to ATP-low cells. Data represent the mean fold increase ±SD over ATP-low 10% cells, n = 3. Unpaired t test, ****p < 0.0001. E Metabolic flux analysis of ATP-high MCF7 cells. The OCR (oxygen consumption rate) and the ECAR (extracellular acidification rate) were determined using the Seahorse XFe96, via metabolic flux analysis. Note that the ATP-high MCF7 cell population shows an increase in both basal and maximal respiration, as well as mitochondrial ATP-production. The ATP-high MCF7 cell population also shows an increase in glycolysis. Cell populations were analyzed 24 h after plating. Data represent the % average fold increase ±SD over ATP-low 10% cells, n = 3. Unpaired t test, ns = not significant, *p < 0.05, **p < 0.005, ***p < 0.0005. F Metabolic flux analysis of ATP-high MDA-MB-231 cells. The OCR (oxygen consumption rate) and the ECAR (extracellular acidification rate) were determined using the Seahorse XFe96, via metabolic flux analysis. Note that the ATP-high MDA-MB-231 cell population shows an increase in both basal and maximal respiration, as well as mitochondrial ATP-production. The ATP-high MDA-MB-231 cell population also shows an increase in glycolytic reserve and glycolytic reserve capacity. Cell populations were analyzed 24 h after plating. Data represent the % average fold increase ±SD over ATP-low 10% cells, n = 3. Unpaired t test, ns = not significant, *p < 0.05, **p < 0.005, ***p < 0.0005. G Cell cycle progression. Note that the ATP-high cell subpopulation is hyper-proliferative, with increases in cell cycle progression, showing a strong shift away from the G0/G1-phase, toward the S-phase and the G2/M-phase. Quantitatively similar results were obtained with MCF7, T47D, MDA-MB-231, and MDA-MB-468 cell lines. In this series of experiments, we used a cut-off of 10% to define the ATP-high and ATP-low cell populations. The percentage of cells in the G0/G1, S, and G2/M phases of the cell cycle are reported. The results are expressed as the mean from three independent experiments, Unpaired t test, **p < 0.005, ***p < 0.0005, ****p < 0.0001. H Tempo-ATP: fluorescent-protein biosensor. Tempo-ATP is a fluorescent-protein biosensor for the detection of cytosolic ATP levels. This protein biosensor was stably expressed in MCF7 cells. The Tempo-ATP-biosensor allows for the measurement of ATP levels in a real-time fashion. As such, high GFP-fluorescent content is a reporter for high ATP content within the cells. The protein sequence of the ATP-binding portion of the biosensor is shown in yellow lettering, while the while GFP-like protein sequence is shown in green. Cells were sorted by GFP content, using flow cytometer (excitation = 517–519 nm; emission = 535 nm). I Tempo-ATP: mammosphere formation. Anchorage-independent growth was quantitated, using the 3D mammosphere formation assay. In this series of experiments, we used a cut-off of 10% to define the GFP-high and GFP-low cell populations. Cells in the monolayer were first sorted for GFP content, by flow cytometry. After cell counting, 5 × 103 cells were seeded in poly-HEMA coated 6-well plates and counted after 5 days. Note that the number of 3D mammospheres formed was nearly fourfold increased in GFP-high MCF7 cells, as compared with GFP-low MCF7 cells, respectively. Data represent the mean fold increase over GFP-low 10% cells ±SD, n = 3. Unpaired t test, ****p < 0.0001. J Tempo-ATP: metabolic analysis. Metabolic profiling of key metabolites in GFP-high and GFP-low TempoATP-MCF7 cells. Luminescence levels were measured in GFP-high and GFP-low TempoATP-MCF7 cell subpopulations, using Promega kits (Cell-Titer-Glo, GSH/GSSG-Glo, NADP-NADPH-Glo, and NAD-NADH-Glo). Cells were sorted by GFP content, using flow cytometry. After cell counting, equal numbers of single cells were then used to evaluate their relative luminescence content. Note that GFP-high MCF7 cells showed a near 2-fold increase in ATP levels; a 2-fold increase in reduced glutathione levels; a ~1.5-fold increase in NADP-NADPH levels; and >2-fold increase in NAD-NADH levels, all relative to GFP-low cells. Data represent the mean fold increase over GFP-low 10% cells ±SD, n = 4. Unpaired t test, **p < 0.005, ***p < 0.0005, ****p < 0.0001. K Tempo-ATP: cell cycle progression. The GFP-high MCF7 cell subpopulation is hyper-proliferative, with increases in cell cycle progression, showing a strong shift away from the G0/G1-phase, towards the S-phase and the G2/M-phase. In this series of experiments, we used a cut-off of 10% to define the GFP-high and GFP-low cell populations. The percentage of cells in the G0/G1, S, and G2/M phases of the cell cycle are reported. The results are expressed as the mean from three independent experiments, Unpaired t test, *** p < 0.0005, ****p < 0.0001.