24.
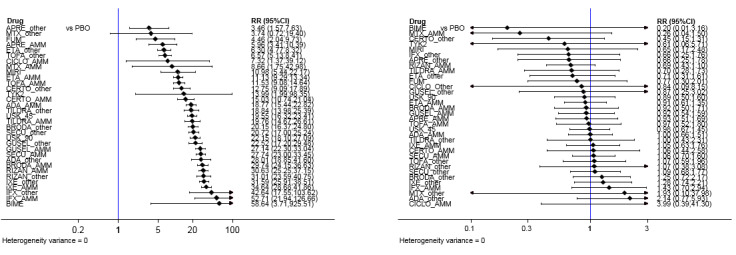
Sensitivity analyses ‐ Interval plot. Network meta‐analysis results for primary outcomes (PASI 90 and serious adverse events, left and right forest plot respectively) for all the interventions depending on the doses: approved dosages versus other dosages
Outcomes were measured at the induction phase (assessment from 8 to 24 weeks after randomisation).
MTX_AMM/Other: methotrexate ≥ 15 mg per week/ < 15 mg per week; CICLO_AMM/Other: ciclosporin ≥ 3 mg/kg/day/<3 mg/kg/day; ACI_AMM/Other: acitretin ≥ 35 mg per day/<35 mg per day; FUM: fumaric acid esters all dosages; APRE_AMM/Other: apremilast 30 mg twice daily/other dosages; TOFA_AMM/Other: tofacitinib 20 mg per day/Other dosages; ETA_AMM/Other: etanercept 50 mg twice a week/Other dosage; IFX_AMM/Other: infliximab 5 mg/kg week 0, 2, 4 every 6 weeks/Other dosages; ADA_AMM/Other: adalimumab 80 mg Week 0, 40 mg Week 1 then 40 mg every other week/Other dosages; CERTO_AMM/Other: certolizumab 400 mg at week 0,2,4 then 400 mg every other week or other dosages/Other dosages; USK 45/90: ustekinumab 45/90 mg; SECU_AMM/Other: secukinumab 300 mg at week 0, 1, 2, 3, and 4 then every 4 weeks or other dosages/other dosages; IXE_AMM/Other: ixekizumab 160 mg at Week then 80 mg every other weeks until week 12 then 80 mg monthly or other dosages; TILDRA_AMM/Other: tildrakizumab 100 mg at week 0 and 4 then every 12 weeks/Other dosages; GUSEL 100: guselkumab 100 mg per injection; BRODA_AMM/Other: brodalumab 210 mg at week 0, 1, 2 then every other weeks/other dosages; RISAN_AMM/Other: risankizumab, S/C, 150 mg (two 75 mg injections) at Week 0, Week 4 and every 12 weeks thereafter/other dosages; TYK2 (Oral Tyrosine kinase 2 inhibitor), MIRI (mirikizumab) and BIME (bimekizumab) (S/C) were grouped in one dosage whatever the dosages.
CI: confidence interval; PASI: Psoriasis Area and Severity Index; RR: risk ratio; AMM: 'approved dosage'
