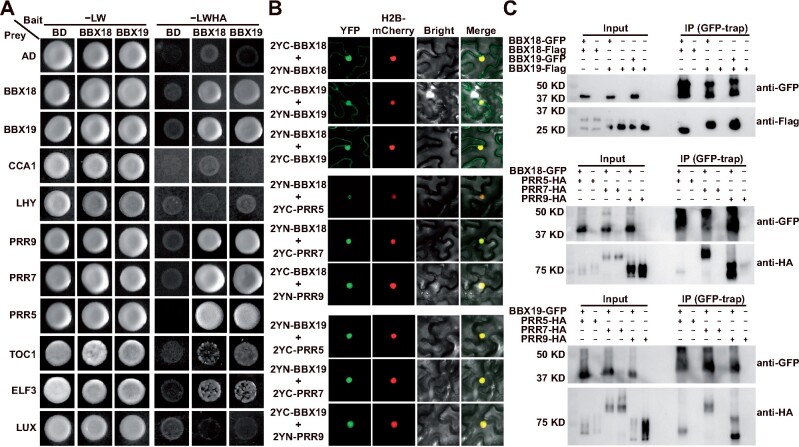
Figure 3.
BBX19 and BBX18 physically interact with PRR proteins in vitro and in vivo. A, Yeast two-hybrid system to screen the interacting proteins of BBX18 and BBX19 among the known clock proteins. AD, activating domain; BD, binding domain; -LW, synthetic dropout medium without leucine and tryptophan; -LWHA, selective medium without leucine, tryptophan, histidine, and adenine. B, BiFC assay showing the interaction between BBX18/19 and PRR proteins predominantly occurred in nucleus. Each protein was tagged with either the N- or C-terminal fragment of YFP as indicated. The fluorescent signal in N. benthamiana epidermal cells was imaged at 48 h after A. tumefaciens-mediated infiltration. C, Co-immunoprecipitation analysis of BBX18, BBX19, and PRRs with transiently expressed proteins in N. benthamiana. Anti-GFP antibody was used for performing immunoprecipitation. The proteins were detected with anti-Flag and anti-HA for immunoblotting as indicated