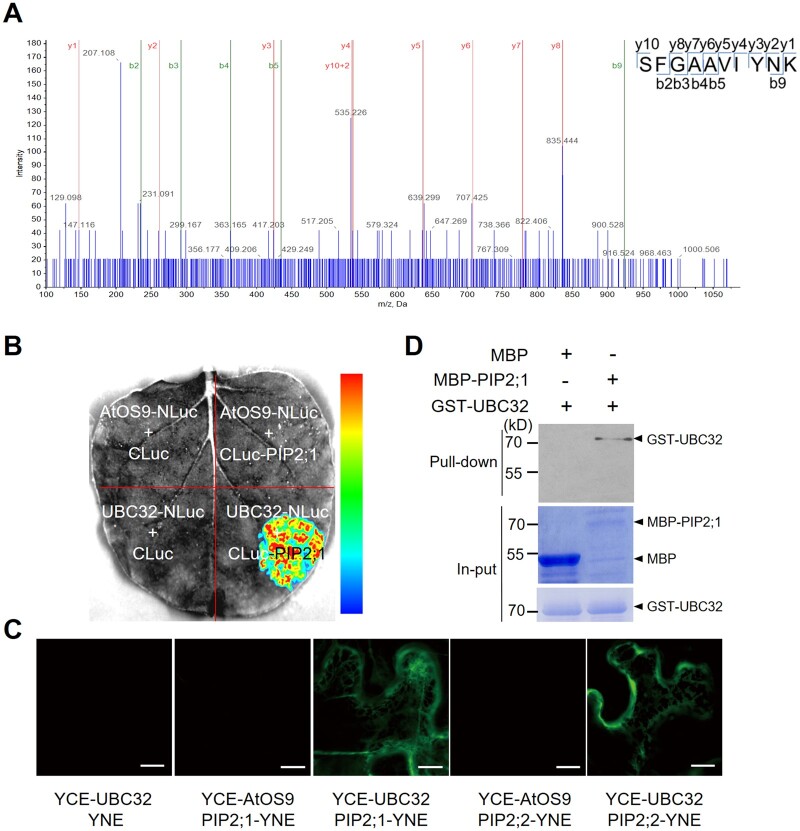
Figure 1.
UBC32 directly interacts with PIP2;1 and PIP2;2. A, PIP2;1 and PIP2;2 were found to interact with UBC32 by mass spectrometry. Total proteins were extracted from UBC32–GFP overexpression plants and immunoprecipitated for mass spectrometry. The SFGAAVIYNK peptide of PIP2;1 and PIP2;2 was identified in the UBC32–GFP immunoprecipitated complex, as illustrated on the right. B, PIP2;1 binds to UBC32 in planta based on LCI experiments. An interaction was detected between UBC32-NLuc and PIP2;1-CLuc, but not in negative controls including AtOS9-NLuc and PIP2;1-CLuc. The pseudocolor bar on the right shows the range of luminescence intensity. C, PIP2;1 interacts with UBC32, as revealed by BiFC assay. The full-length coding sequence of UBC32 was cloned into pSPYCE (M) and the full-length coding sequences of PIP2s were inserted into pSPYNE (R) 173. AtOS9 was used as a negative control in this BiFC assay, bar = 10 μm. D, PIP2;1 interacts with UBC32 in a pull-down assay. E. coli-expressed MBP, MBP-PIP2;1, and GST-UBC32 were used in the pull-down assay. Equal amounts of MBP and MBP-PIP2;1 bound to amylose resin were incubated with 2 μg GST-UBC32. Pull-down products were detected using anti-GST antibody. The arrowheads on the right indicate the positions of full-length GST/MBP-tagged proteins.