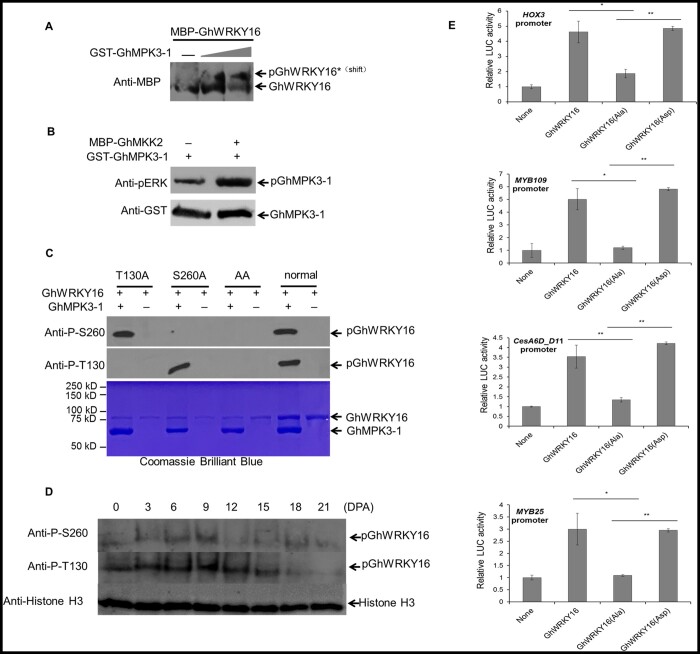
Figure 6.
Phosphorylation of GhWRKY16 by GhMPK3-1 enhances its transcriptional activation of downstream target genes. A, Immunoblotting analysis of in vitro phosphorylation of GhWRKY16 by recombinant GhMPK3-1 with SuperSep Phos-tagTM SDS–PAGE. Phosphorylated GhWRKY16 (pWRKY16) migrates more slowly in the gel. Gray triangle, increasing amount of MBP-GhWRKY16. B, In vitro kinase activity assay of GhMPK3-1 and GhMKK2. Phosphorylation of GhMPK3-1 by recombinant GhMKK2 was detected by immunoblotting with the anti-pERK antibody (Cell Signaling, Danvers, MA, USA), recognizing phosphorylated Extracellular Regulated protein Kinases (top), and anti-GST antibody to quantify GhMPK3-1 (bottom). C, Identification of phosphorylation sites in GhWRKY16 by in vitro kinase activity assay. T130A, GhWRKY16T130A; S260A, GhWRKY16S260A; AA, GhWRKY16T130A/S260A. Upper, immunoblot analysis; lower, SDS–PAGE gel stained by Coomassie Brilliant Blue for loading. Anti-P-T130, anti-phosphorylated WRKY16 Thr130 antibody; Anti-P-S260, anti-phosphorylated WRKY16 Ser260 antibody. D, Phosphorylation levels of GhWRKY16 in cotton fibers at different developmental stages. E, Effects of GhWRKY16 phosphorylation on the transcription of downstream target genes by dual LUC reporter assay. LUC activity was normalized to REN activity, with LUC/REN activity from the control without effector (None) set to 1. GhWRKY16, wild-type GhWRKY16; GhWRKY16(Ala), GhWRKY16T130A/S260A mutant; GhWRKY16(Asp), GhWRKY16 phospho-mimic. Error bars represent sd of three biological replicates. **P <0.01 by t test between phosphorylated GhWRKY16 and nonphosphorylated GhWRKY16(Ala)