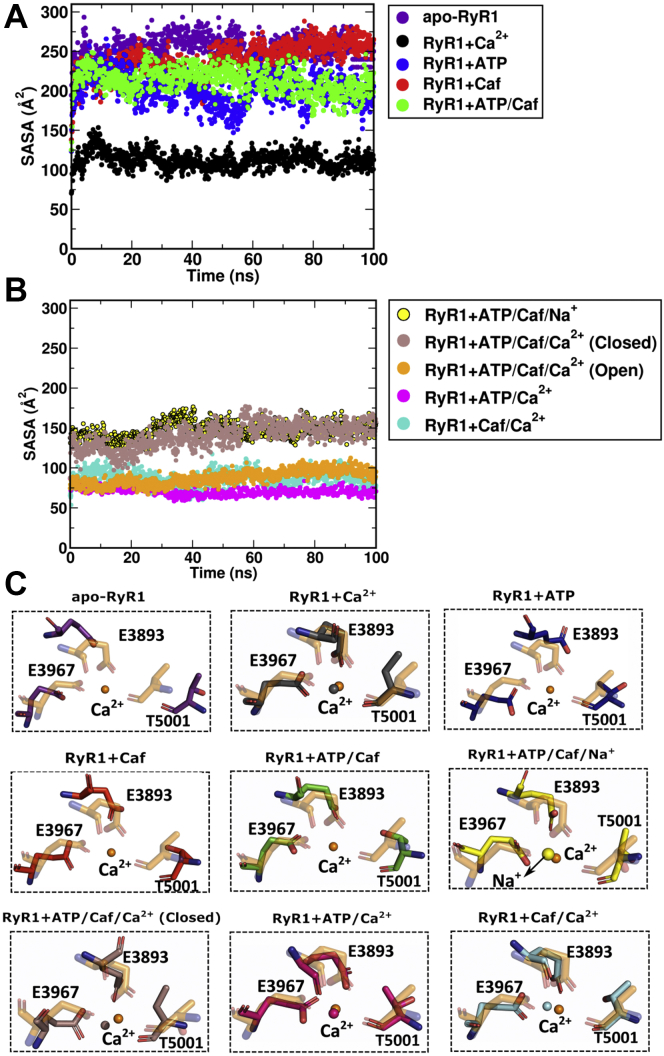
Figure 4.
Quantification of size and dynamics of the Ca2+-binding site in different RyR1 functional states. To estimate the size of Ca2+-binding site, SASA of Ca2+-binding site residues Glu-3893, Glu-3967, and Thr-5001 was measured in different RyR1 systems. Evaluation of SASA with respect to the simulation time suggests that the Ca2+-binding site exhibits wide and expanded conformation of SASA in the absence of Ca2+ (plot A). The binding of Ca2+ along with ATP, caffeine, or both displayed stable and closed conformation of Ca2+-binding site of SASA (plot B). C, structural superposition of average Ca2+-binding sites extracted from last 20 ns of respective simulation trajectories to the average conformation of Ca2+-binding site of open RyR1 + ATP/Caf/Ca2+ (orange color). The Ca2+-binding site residues are shown in stick representation, and bound ions (Ca2+/Na+) are shown as spheres. The color code for Ca2+-binding site is as follows: apo-RyR1 (purple), RyR1 + Ca2+ (black), RyR1 + ATP (blue), RyR1 + Caf (red), RyR1 + ATP/Caf (green), RyR1 + ATP/Caf/Na+ (yellow), closed RyR1 + ATP/Caf/Ca2+ (brown), open RyR1 + ATP/Caf/Ca2+ (orange), RyR1 + ATP/Ca2+ (magenta), and RyR1 + Caf/Ca2+ (cyan). To avoid complexity, we maintained same color for Ca2+-binding site and corresponding bound Ca2+/Na+ ions. RyR1, ryanodine receptor type 1; SASA, solvent-accessible surface area.