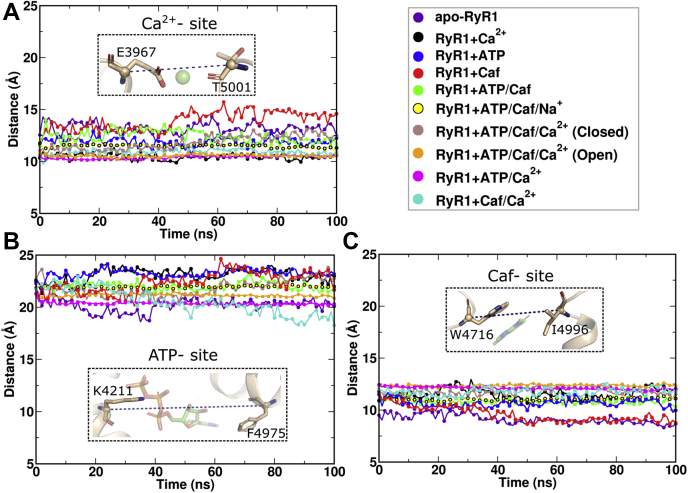
Figure 5.
Quantification of ligand-binding site dimensions to evaluate the dynamics of Ca2+-, ATP-, and caffeine (Caf)-binding sites. Distance variations between backbone atoms of amino acid residues present on the opposite sides of ligand-binding sites with respect to simulation time. We considered the backbone Cα atoms of (A) Glu-3967 and Thr-5001, (B) Lys-4211 and Phe-4975, and (C) Trp-4716 and Ile-4996 to evaluate the dynamics of Ca2+-, ATP-, and Caf-binding sites, respectively. The comparative analysis shows that Ca2+-binding site is dynamic and unstable in the absence of Ca2+. The ATP-binding pocket is constricted in apo-RyR1, RyR1 + ATP/Ca2+, and RyR1 + Caf/Ca2+ systems possibly because of structural rearrangements in TaF and CTD. The Caf-binding pocket is constricted and flexible in apo-RyR1 and RyR1 + Caf systems. The residues used for the distance measurement are shown as sticks in the insets. ATP and Caf are shown in stick representation, and Ca2+ is shown in sphere representation. CTD, C-terminal domain; RyR1, ryanodine receptor type 1.