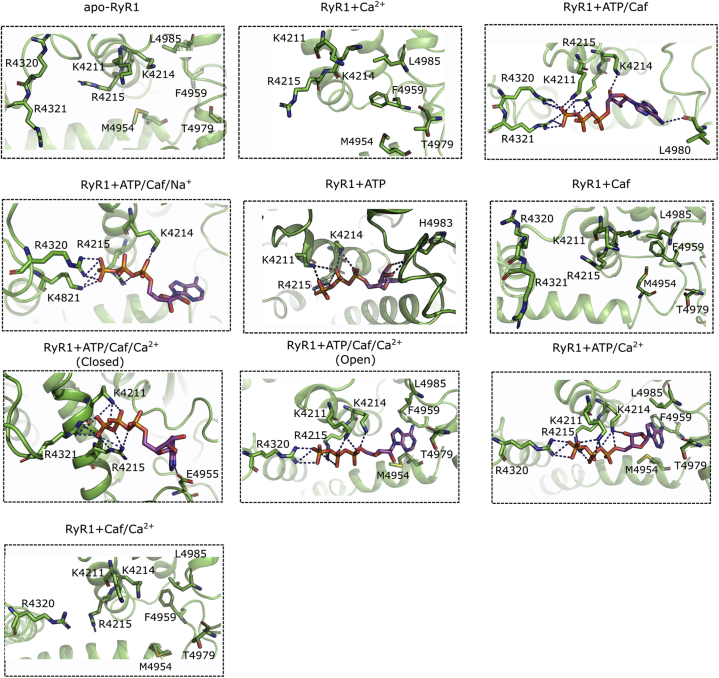
Figure 6.
Evaluation of ATP-binding site interactions in different RyR1 functional states. Interactions of ATP molecule residues with pocket lining amino acid in different RyR1 functional states were evaluated by extracting average structures from last 20 ns of respective trajectories. Our comparative analysis suggests that ATP has differential interactions in open and closed RyR1 states. Furthermore, the ATP-site interactions were modulated by the presence or absence of Ca2+ and/or Caf. The protein is shown as cartoon in green with ATP-binding site residues in stick representation. ATP molecule is rendered in magenta stick representation, and H-bond interactions are shown as blue dotted lines. RyR1, ryanodine receptor type 1.