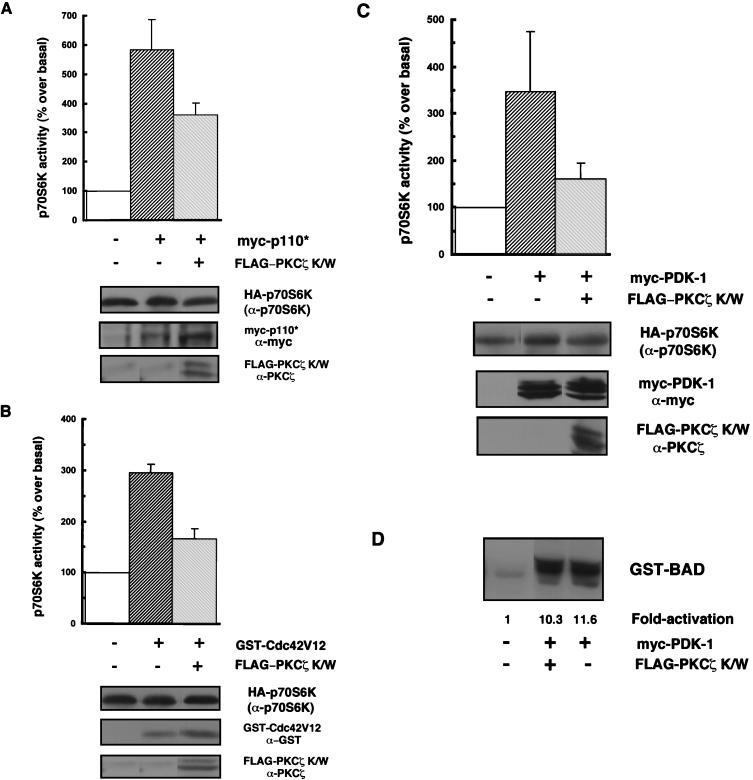
FIG. 3.
Kinase-inactive PKCζ antagonizes p70S6K activation by various stimuli. (A, B, and C) 293 cells were cotransfected with HA-p70S6K (0.25 to 5 μg) along with 2 μg of myc-p110* (A) or myc-PDK-1 (C) or with 3 μg of GST-Cdc42V12 (B) in the presence or absence of 8 μg of PKCζ K/W, or HA-p70S6K was cotransfected only with an empty vector (pCMV5). Cells were serum starved for 24 h prior to harvesting. myc-p110*, myc-PDK-1, GST-Cdc42V12, and FLAG-PKCζ protein expression levels were monitored in whole-cell lysates by immunoblotting using anti-myc, anti-GST, or anti-PKCζ antibodies, respectively (lower panels). HA-p70S6K protein expression (top of three lower panels) and activity were monitored and quantitated as described in the legend to Fig. 2B. The bar graphs represent p70S6K activity expressed as a percentage of basal p70S6K activity. Results are expressed as the mean percentage over the basal level ± the standard error of the mean (n = 3–6). Blots are representative of independent experiments. (D) Equal amounts of HA-Akt were immunoprecipitated from lysates from 293 cells which were cotransfected with HA-Akt (1 μg) and PDK-1 (2 μg) with or without FLAG-PKCζ K/W (8 μg) or with HA-Akt and an empty vector, and HA-Akt activity was assayed by in vitro phosphorylation of GST-BAD as described in Materials and Methods and is expressed as fold activation over the basal level.