Abstract
Background/Aim: To report the feasibility and oncological outcomes in breast cancer patients treated with a short hypofractionated radiotherapy schedule. Patients and Methods: We evaluated 380 breast cancer patients treated with ten daily fractions of radiotherapy up to 39 Gy on tumor bed. Primary endpoint was local relapse rate (LRR). Secondary endpoints were overall survival (OS) and metastasis-free survival (MFS). Results: The median follow up was 5.0 years. Two- and 5-year LRR rates were 0.2 and 2%, respectively. Two- and 5-year MFS rates were 96.1% and 90.5%, respectively. Two and 5-year OS rates were 97.4% and 95%, respectively. Conclusion: This short schedule may represent an alternative option to standard mild hypofractionated radiotherapy in breast cancer patients due to its excellent feasibility and very low recurrence rate.
Keywords: Hypofractionated radiotherapy, early breast cancer, adjuvant treatment, local relapse, toxicity
Adjuvant whole breast radiotherapy (RT) after breast conserving surgery (BCS) is the standard treatment in early breast cancer (1). Several data have demonstrated that it reduces the risk of local recurrence and provides a beneficial effect on overall survival with excellent cosmetic results (1). Conventional adjuvant RT fractionation consisted of 50 Gy in 25 fractions delivered over 5 weeks followed by a boost dose of 10-16 Gy or not to the tumor bed (1). In the last decades, alternative radiotherapy schedules were investigated and randomized controlled trials (RCT) have largely tested the efficacy and safety of a hypofractionated RT schedule compared to a conventional regimen (2,3). The increasing interest in reducing the duration of RT with hypofractionated regimens is due to different reasons: the radiobiological one is based on a relative low α/β ratio - for breast cancer cells it is estimated to be 4 and then quite close to those of late reacting normal tissues (4); this observation means that breast cancer appears to be sensitive to the fraction size (5). Moreover, it has to be considered that RT is typically delivered after surgery and chemotherapy and is the last part of a multimodality treatment that could even be longer than six months. From this point of view, hypofractionation RT has also potential advantages for patients as it reduces overall treatment time and improves quality of life. Moreover, it leads to a reduction of costs for the healthcare system compared to a standard RT schedule (6). The aim of this work was to report the feasibility and oncological outcomes in a large group of breast cancer patients treated with conservative surgery followed by adjuvant, whole breast intensified hypofractionated regimen, scheduled in 10 fractions.
Patients and Methods
From November 2012 to January 2016, 380 consecutive early breast cancer patients, treated with BCS and adjuvant RT, were analyzed. Written informed consent to undergo RT was obtained by all patients. The study was approved by Ethics Committee of IRCCS Policlinico San Martino Hospital of Genoa (n˚ 45/2020).
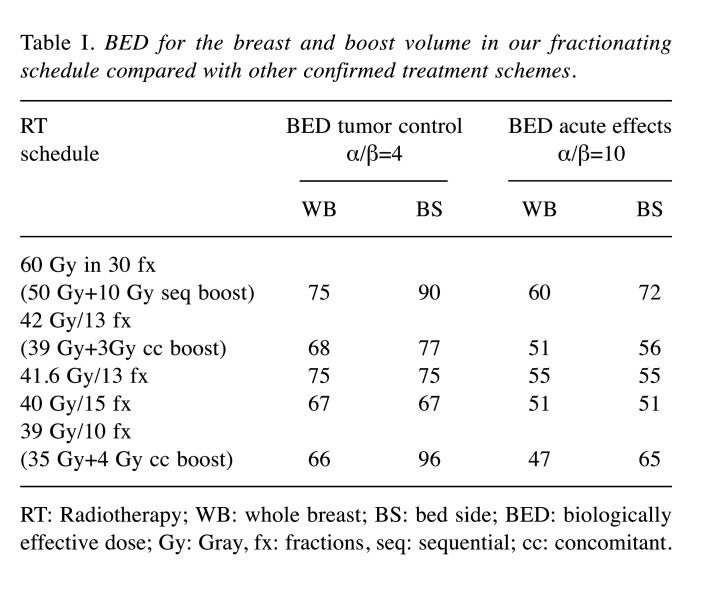
Radiation treatment. The eligibility criteria for enrollment were the following: age>44 years old, histological diagnosis of breast carcinoma, stage pTis-T2, pN0-N1, M0 according to American Joint Committee on Cancer Staging Manual, Eighth Edition (2017). Exclusion criteria were: tumor greater than 5 cm in its largest dimension, 3 or more positive nodes, presence of serious co-morbidities that could preclude radiotherapy, previous thoracic irradiation, male patients. The RT regimen was planned either immediately after conservative surgery in low-risk patients or sequentially after chemotherapy in patients at high-risk of disease progression. Each patient received whole breast radiotherapy (WBRT) in 10 fractions of 3.5 Gy (4 fractions a week) to a total dose of 35 Gy. Twice a week, immediately after WBRT, a simultaneous photon boost of 100 cGy was delivered to the lumpectomy area; the total dose to the tumor bed was 38 Gy in case of negative surgical margins or 39 Gy with close or positive margins. No patients underwent nodal irradiation. Using the linear-quadratic cell survival model and assuming an a/β ratio of 4 Gy for tumor response (4) and 10 Gy for acute responding normal tissues (7), we calculated BED for the breast and boost volume in our fractionating schedule, in comparison with the standard and other confirmed treatment schemes (Table I). Definition of target volumes, organ at risks (OARs), and treatment planning were previously described (8).
Table I. BED for the breast and boost volume in our fractionating schedule compared with other confirmed treatment schemes.
RT: Radiotherapy; WB: whole breast; BS: bed side; BED: biologically effective dose; Gy: Gray, fx: fractions, seq: sequential; cc: concomitant.
Follow up. Patients were evaluated at the end of treatment and six months after the end of radiotherapy for radiotherapy-induced normal tissue effects, and then reviewed every year for tumor relapse. Acute toxicity was assessed using the Common Terminology Criteria for Adverse Events (CTCAE version 4.03) (9), and late toxicity using Radiation Therapy Oncology Group (RTOG)/European Organization for Research and Treatment of Cancer (EORTC) scale for radiation-related toxicity (10).
Endpoints. The primary endpoint of this analysis was the local relapse rate (LRR). Secondary endpoints were acute and late toxicity, overall survival (OS) and metastasis-free survival (MFS).
Statistical analysis. Age was summarized with mean and standard deviation (SD). Tumor size and follow-up periods were summarized with median and inter quartile range (IQR) due to their skewed distribution. Categorical variables were summarized with count and percentages. Cox model and Kaplan-Meier method were used to estimate OS and MFS and reported as probability of being event free at 5 years, 95% confidence interval (95%CI), absolute number of events, and number of patients at risk; a competing risks model was used in the estimation of local disease control, considering death as a concurrent event. R software 3.6.0 was used for statistical analysis.
Results
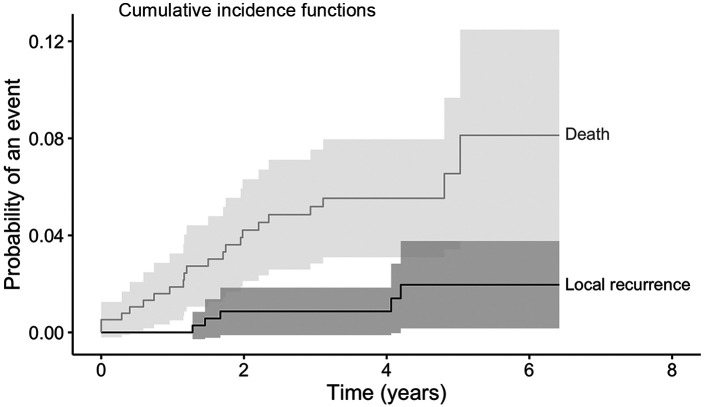
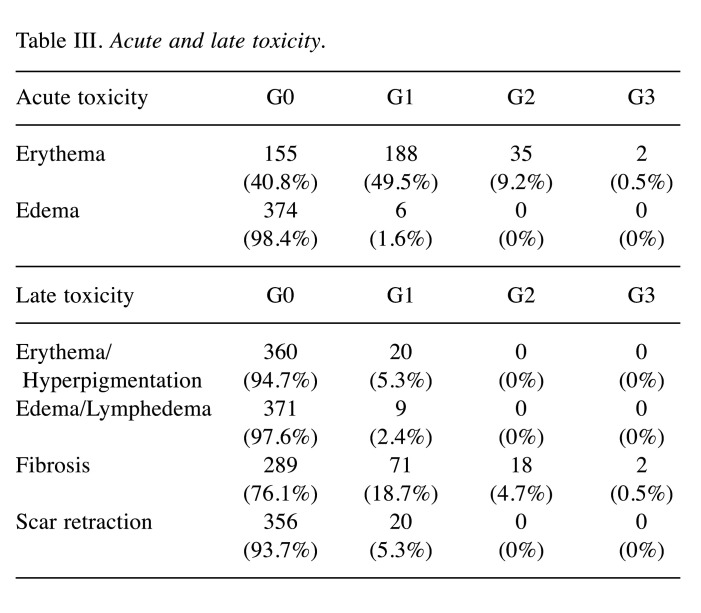
A total of 380 patients were analyzed. Median follow-up was 5.0 years (IQR=4.4-5.6). Patient’s characteristics are described in Table II. Mean age was seventy years old (range=40-89 years old). About 14% of the patients had pTis tumor, almost 70% had pT1, and about 17% had pT2. Most of the patients were pN0 (73.4%). 84% of margins were negative after breast conserving surgery. Also, 77.6% and 16.6% of patients received adjuvant hormonal therapy and chemotherapy, respectively. All patients completed the planned RT schedule. At the time of the analysis, five patients had experienced a locoregional recurrence, three patients a nodal recurrence, one patient a breast recurrence, and one patient both breast and nodal recurrence. Twenty-two deceased patients: seven due to metastasis and fifteen due to non-cancer-related causes. Seventeen patients developed distant metastases, mainly in the liver, bone, and lungs. Cumulative incidence of local relapses at 2 and 5 years was 0.8% (95%CI=0.01-1.8, number of relapse events 3) and 2.0% (95%CI=0.2-4.0, number of relapse events 5), respectively (Figure 1). OS at 2 and 5 years was 97.4% (95%CI=95.8-99, number of events 10, number at risk 369) and 95% (95%CI=92.8-97.2, number of events 19, number at risk 197), respectively (Figure 2). MFS at 2 and 5 years was 96.1% (95%CI=94.1-98.1, number of metastasis or death events 29, number at risk 314) and 90.5% (95%CI=87.0-94.0, number of metastasis or death events 29, number at risk 65), respectively (Figure 3). In regard to acute toxicity, at the end of the treatment, 155 (40.8%) patients presented G0 erythema, 188 (49.5%) G1 erythema, 35 (9.2%) G2 erythema, and 2 (0.5%) G3 erythema. Only six patients presented mild edema (Table III). At six months, 71 patients presented G1 fibrosis, 18 G2 fibrosis, and only two patients presented G3 fibrosis. In twenty cases there was a slight erythema or hyperpigmentation; in twenty other cases there was a slight/modest scar retraction. Edema and lymphedema were present in nine cases (Table III).
Table II. Patient characteristics.
YRS: Years; N: number; MM: millimeter; CT: chemotherapy; OT: hormonal therapy; ER: estrogen; PgR: progesterone. *not evaluable in 48 patients; **not reported on histological examination (ex. in pTis tumor); ***not evaluable in 65 patients (due to the lack of one or more biological data).
Figure 1. Cumulative incidence of local recurrence and death. Kaplan-Meier curve describing the risk of local recurrence and the risk of death.
Figure 2. Kaplan-Meier curve describing overall survival.
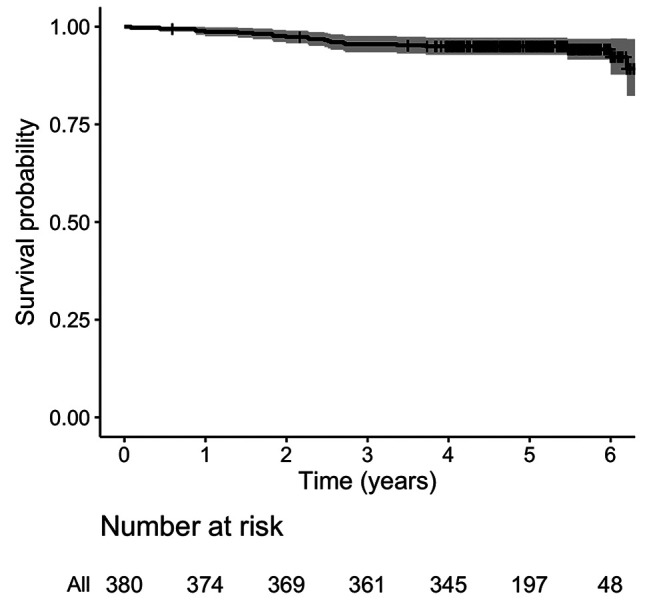
Figure 3. Kaplan-Meier curve describing metastasis-free survival.
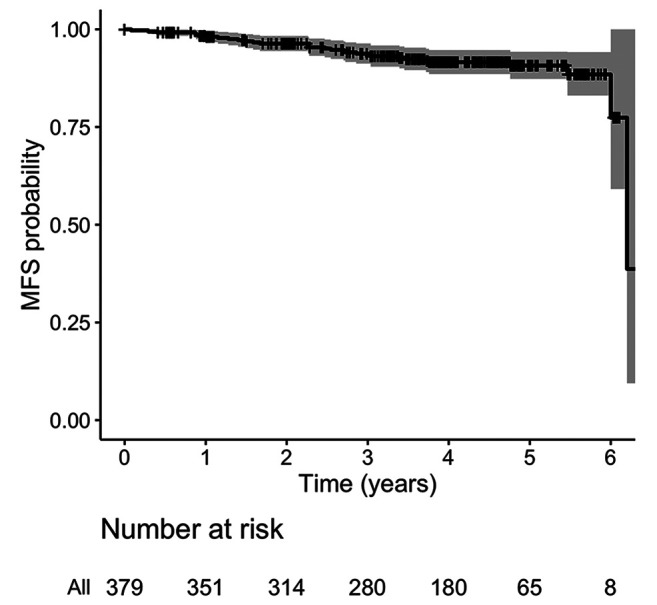
Table III. Acute and late toxicity.
Discussion
Whole breast RT is the standard of care in patients with early cancer after BCS (1). In 2015, a meta-analysis by Budach et al. identified four RCT (START Pilot, START A, START B and Ontario) that evaluated hypofractionated RT compared to conventional fractionated RT. All these trials concluded that hypofractionated RT can be safely used in early breast cancer patients as it leads to similar oncological outcomes with no increase in late toxicity (11). More recently, a 2016 Cochrane review on hypofractionated RT for early breast cancer confirmed the same conclusions both for oncological results and for acute and late toxicities and quality of life (12). Today, therefore, these data allow us to consider hypofractionation as an effective option in adjuvant breast RT (13). In fact, RCT as START A have shown that 41.6 Gy/13 fractions or 39 Gy/13 fractions are similar to the control regimen of 50 Gy/25 fractions in terms of local-regional tumor control and late normal tissue effects; similarly, the START trial B, which randomized 2,215 women with early breast cancer (pT1-3a pN0-1 M0) to a radiation scheme of 40 Gy/15 fractions or 50 Gy/25 fractions, reported a rate of local-regional tumor relapse at 5 years of 2.2% in the 40 Gy group and 3.3% in the 50 Gy group after a median follow-up of 6.0 years (14). An update at 10 years after these two English trials showed that local-regional relapse did not differ significantly between the hypofractionated schemes and standard regimen groups (3). In our analysis, we proposed this new intensified scheme that showed a very low mid-term recurrence rate similar to those reported with other consolidated fractionations. A similar ten fraction schedule was explored by Trovò et al. using partial breast irradiation (PBI). Outcomes recently published (15) showed excellent results in outcome and feasibility. Although results from a randomized trial (16) seem to establish the role of PBI, this modality is not largely adopted and WBI remains the most adopted in clinical practice. Comparing our 5 years results obtained by short hypofractionated WBI with those obtained from the START-A and START-B trials the LRR are similar: 3.5% after 41.6 Gy, 5.2% after 39 Gy (START-A, 13 fractions), 2.2% after 40 Gy (START-B, 15 fractions) and 2% after 35 Gy (Genoa Trial, 10 fractions). Stronger hypofractionated schedules were recently introduced in the FAST and FAST Forward trials, two 3-arm randomized control phase III trials. The first compared standard fractionation (50 Gy/25 fractions) with 5.7/6 Gy×5 fractions delivered once a week and reported incidence rates for ipsilateral breast events of 0.7% at 5 years (even if the trial was not powered for comparison of recurrence rates, primary endpoint changed in breast appearance at 2 and 5 years) (17). The FAST Forward trial evaluated ultra hypofractionated schedules (5.2/5.4 Gy×5 consecutive fractions vs. START B arm) with an estimated cumulative incidence rate of ipsilateral breast tumor relapse at 5 years of 2.1% for the standard arm, 1.7% for 27 Gy, and 1.4% for 26 Gy (no difference) (18). In our center, we have used hypofractionated RT for breast cancer for several years; a hypofractionation of 46 Gy in 20 fractions (19,20) and 39 Gy in 13 fractions (8) have been carried out since 2007 and 2008, respectively, showing a reasonable good feasibility in terms of safety, efficacy, acute and subacute toxicity, in line with the British studies. Only in elderly women (over 70 years old) we are now adopting an extreme hypofractionation scheme (5.7 Gy×5 weekly fractions) (21) according to the latest NCCN guidelines that suggest this schedule (28.5 Gy in 5 fractions once a week) only for selected patients (22). We underline that long-term data on toxicity from extreme hypofractionation schedules were not well defined; both in the FAST and FAST Forward trials moderate and marked late effects were increased in the experimental arm. Moreover, in the FAST Forward trial, the relative risk for any moderate and marked late effects increased over time, indicating that longer follow-up is necessary to evaluate the long-term safety of this regimen (23). In regard to acute and late toxicities, our analysis showed low grade side effects to the skin and subcutaneous tissues. At the end of the treatment, about half of the patients presented mild erythema; only two reported a grade 3 skin toxicity. Six months after the end of the treatment, 5% of the patients had mild hyperpigmentation and about 18% mild fibrosis. Only 0.5% reported G3 fibrosis.
Despite the limitations of this study due to its retrospective design, the initial analysis of the late effects appears promising. This seems in line with the results of other important studies in the literature (24-26). Another interesting point is that our scheme provides the administration of a simultaneous boost in all patients (27); this is different from those of the aforementioned randomized trials where a boost dose was not administered or it was delivered with a standard fractionation; even in the FAST Forward trial a sequential boost with 5-8 fractions of 2 Gy was applied, but this led to double the overall treatment time. It is well known that tumor bed is the area at highest risk for persistent microscopic disease and a local dose escalation has proven to decrease the in-breast recurrence rates most effectively, especially in younger women (28). Moreover, we chose to adjust boost dose according to the margin status (4 Gy in 4 fractions for patients with positive or close margins and 3 Gy in 3 fractions in negative margins) in order to reduce the risk of local relapse.
This study also has other limitations, including the lack of a comparison arm in the analysis; however, to date, this hypofractionated schedule can be easily prescribed and delivered in early breast cancer both for radiobiological rationale, technological innovations, and logistic reasons. In conclusion, this intensified radiation schedule, delivered in ten fractions, offers excellent outcome and may provide an alternative option to conventional WBI, providing similar results in terms of disease control with a satisfactory quality of life for women during and after treatment.
Conflicts of Interest
The Authors declare that they have no conflicts of interest.
Authors’ Contributions
LB: wrote the paper, and reviewed final manuscript. LT: data collection. FC and SA: review and editing of final manuscript. Provided insight into how results relate to treatment planning. LC: data analysis, ran all statistical tests. RC, AF and MG: review and editing of final manuscript and provided insight into how results relate to radiation oncology. DF and PF: review and editing of final manuscript and provided insight into how results relate to surgical oncology. All Authors approved the final version of the manuscript.
References
- 1.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) , Darby S, McGale P, Correa C, Taylor C, Arriagada R, Clarke M, Cutter D, Davies C, Ewertz M, Godwin J, Gray R, Pierce L, Whelan T, Wang Y, Peto R. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378(9804):1707–1716. doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whelan TJ, Pignol JP, Levine MN, Julian JA, MacKenzie R, Parpia S, Shelley W, Grimard L, Bowen J, Lukka H, Perera F, Fyles A, Schneider K, Gulavita S, Freeman C. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med. 2010;362(6):513–520. doi: 10.1056/NEJMoa0906260. [DOI] [PubMed] [Google Scholar]
- 3.Haviland JS, Owen JR, Dewar JA, Agrawal RK, Barrett J, Barrett-Lee PJ, Dobbs HJ, Hopwood P, Lawton PA, Magee BJ, Mills J, Simmons S, Sydenham MA, Venables K, Bliss JM, Yarnold JR, START Trialists’ Group The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol. 2013;14(11):1086–1094. doi: 10.1016/S1470-2045(13)70386-3. [DOI] [PubMed] [Google Scholar]
- 4.Owen JR, Ashton A, Bliss JM, Homewood J, Harper C, Hanson J, Haviland J, Bentzen SM, Yarnold JR. Effect of radiotherapy fraction size on tumour control in patients with early-stage breast cancer after local tumour excision: long-term results of a randomised trial. Lancet Oncol. 2006;7(6):467–471. doi: 10.1016/S1470-2045(06)70699-4. [DOI] [PubMed] [Google Scholar]
- 5.Hennequin C, Guillerm S, Quero L. Rationale for hypofractionation. Cancer Radiother. 2019;23(6-7):500–502. doi: 10.1016/j.canrad.2019.07.156. [DOI] [PubMed] [Google Scholar]
- 6.Rivera S, Hannoun-Lévi JM. Hypofractionated radiation therapy for invasive breast cancer: From moderate to extreme protocols. Cancer Radiother. 2019;23(8):874–882. doi: 10.1016/j.canrad.2019.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Fowler JF. 21 years of biologically effective dose. Br J Radiol. 2010;83(991):554–568. doi: 10.1259/bjr/31372149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corvò R, Ricchetti F, Doino D, Torielli P, Agostinelli S, Cavagnetto F, Giannelli F, D’Alonzo A, Vagge S, Belgioia L, Guenzi M. Adjuvant hypofractionated radiotherapy with weekly concomitant boost for women with early breast cancer: the clinical experience at Genoa university. Anticancer Res. 2010;30(11):4749–4753. [PubMed] [Google Scholar]
- 9.Common Terminology Criteria for Adverse Events (CTCAE) | Protocol Development | CTEP. Available at: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40. [Last accessed on January 15, 2020]
- 10.RTOG/EORTC Late Radiation Morbidity Scoring Schema. Available at: https://www.rtog.org/ResearchAssociates/AdverseEventReporting/RTOGEORTCLateRadiationMorbidityScoringSchema.aspx. [Last accessed on January 15, 2020]
- 11.Budach W, Bölke E, Matuschek C. Hypofractionated radiotherapy as adjuvant treatment in early breast cancer. A review and meta-analysis of randomized controlled trials. Breast Care (Basel) 2015;10(4):240–245. doi: 10.1159/000439007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hickey B, James M, Lehman M, Hider P, Jeffery M, Francis D, See A. Hypofractionated radiation therapy for early breast cancer. Cochrane Database of Systematic Reviews. 2021 doi: 10.1002/14651858.CD003860.pub4. [DOI] [Google Scholar]
- 13.Smith B, Bellon J, Blitzblau R, Freedman G, Haffty B, Hahn C, Halberg F, Hoffman K, Horst K, Moran J, Patton C, Perlmutter J, Warren L, Whelan T, Wright J, Jagsi R. Radiation therapy for the whole breast: Executive summary of an American Society for Radiation Oncology (ASTRO) evidence-based guideline. Practical Radiation Oncology. 2020;8(3):145–152. doi: 10.1016/j.prro.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 14.START Trialists’ Group , Bentzen SM, Agrawal RK, Aird EG, Barrett JM, Barrett-Lee PJ, Bliss JM, Brown J, Dewar JA, Dobbs HJ, Haviland JS, Hoskin PJ, Hopwood P, Lawton PA, Magee BJ, Mills J, Morgan DA, Owen JR, Simmons S, Sumo G, Sydenham MA, Venables K, Yarnold JR. The UK Standardisation of Breast Radiotherapy (START) Trial A of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet Oncol. 2008;9(4):331–341. doi: 10.1016/S1470-2045(08)70077-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vinante L, Avanzo M, Furlan C, Fiorica F, Perin T, Militello L, Spazzapan S, Berretta M, Jena R, Stancanello J, Piccoli E, Mileto M, Micheli E, Roncadin M, Massarut S, Trovò M. Ten daily fractions for partial breast irradiation. Long-term results of a prospective phase II trial. Breast J. 2019;25(2):243–249. doi: 10.1111/tbj.13195. [DOI] [PubMed] [Google Scholar]
- 16.Meattini I, Marrazzo L, Saieva C, Desideri I, Scotti V, Simontacchi G, Bonomo P, Greto D, Mangoni M, Scoccianti S, Lucidi S, Paoletti L, Fambrini M, Bernini M, Sanchez L, Orzalesi L, Nori J, Bianchi S, Pallotta S, Livi L. Accelerated partial-breast irradiation compared with whole-breast irradiation for early breast cancer: Long-term results of the randomized phase III APBI-IMRT-florence trial. J Clin Oncol. 2020;38(35):4175–4183. doi: 10.1200/JCO.20.00650. [DOI] [PubMed] [Google Scholar]
- 17.Brunt AM, Haviland JS, Sydenham M, Agrawal RK, Algurafi H, Alhasso A, Barrett-Lee P, Bliss P, Bloomfield D, Bowen J, Donovan E, Goodman A, Harnett A, Hogg M, Kumar S, Passant H, Quigley M, Sherwin L, Stewart A, Syndikus I, Tremlett J, Tsang Y, Venables K, Wheatley D, Bliss JM, Yarnold JR. Ten-year results of FAST: A randomized controlled trial of 5-fraction whole-breast radiotherapy for early breast cancer. J Clin Oncol. 2020;38(28):3261–3272. doi: 10.1200/JCO.19.02750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murray Brunt A, Haviland JS, Wheatley DA, Sydenham MA, Alhasso A, Bloomfield DJ, Chan C, Churn M, Cleator S, Coles CE, Goodman A, Harnett A, Hopwood P, Kirby AM, Kirwan CC, Morris C, Nabi Z, Sawyer E, Somaiah N, Stones L, Syndikus I, Bliss JM, Yarnold JR, FAST-Forward Trial Management Group Hypofractionated breast radiotherapy for 1 week versus 3 weeks (FAST-Forward): 5-year efficacy and late normal tissue effects results from a multicentre, non-inferiority, randomised, phase 3 trial. Lancet. 2020;395(10237):1613–1626. doi: 10.1016/S0140-6736(20)30932-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guenzi M, Vagge S, Azinwi NC, D’Alonzo A, Belgioia L, Garelli S, Gusinu M, Corvò R. A biologically competitive 21 days hypofractionation scheme with weekly concomitant boost in breast cancer radiotherapy feasibility acute sub-acute and short term late effects. Radiat Oncol. 2010;5:111. doi: 10.1186/1748-717X-5-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guenzi M, Bonzano E, Corvò R, Merolla F, Pastorino A, Cavagnetto F, Garelli S, Cutolo CA, Friedman D, Belgioia L. Comparison of local recurrence among early breast cancer patients treated with electron intraoperative radiotherapy vs. hypofractionated photon radiotherapy an observational study. Front Oncol. 2018;8:207. doi: 10.3389/fonc.2018.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonzano E, Belgioia L, Polizzi G, Siffredi G, Fregatti P, Friedman D, Garelli S, Gusinu M, Vaccara EML, Guenzi M, Corvò R. Simultaneous integrated boost in once-weekly hypofractionated radiotherapy for breast cancer in the elderly: Preliminary evidence. In Vivo. 2019;33(6):1985–1992. doi: 10.21873/invivo.11694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.NCCN Guidelines. Available at: http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. [Last accessed on June 14 2021]
- 23.Krug D, Baumann R, Combs SE, Duma MN, Dunst J, Feyer P, Fietkau R, Haase W, Harms W, Hehr T, Piroth MD, Sedlmayer F, Souchon R, Strnad V, Budach W, Breast Cancer Expert Panel of the German Society of Radiation Oncology (DEGRO) Moderate hypofractionation remains the standard of care for whole-breast radiotherapy in breast cancer: Considerations regarding FAST and FAST-Forward. Strahlenther Onkol. 2021;197(4):269–280. doi: 10.1007/s00066-020-01744-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Felice F, Ranalli T, Musio D, Lisi R, Rea F, Caiazzo R, Tombolini V. Relation between hypofractionated radiotherapy, toxicity and outcome in early breast cancer. Breast J. 2017;23(5):563–568. doi: 10.1111/tbj.12792. [DOI] [PubMed] [Google Scholar]
- 25.Iatì G, Pontoriero A, Mondello S, Santacaterina A, Platania A, Frosina P, Raso MM, Aiello D, Arcudi A, Arena G, Marino G, Mazzei M, Rifatto C, Risoleti E, Runco R, Sansotta G, Delia P, Sindoni A, Pergolizzi S. Nodal ratio as a prognostic factor in patients with four or more positive axillary nodes treated with breast-conserving therapy and regional nodal irradiation. Anticancer Res. 2016;36(7):3549–3554. [PubMed] [Google Scholar]
- 26.Palumbo I, Mariucci C, Falcinelli L, Perrucci E, Lancellotta V, Podlesko AM, Marcantonini M, Saldi S, Bini V, Aristei C. Hypofractionated whole breast radiotherapy with or without hypofractionated boost in early stage breast cancer patients: a mono-institutional analysis of skin and subcutaneous toxicity. Breast Cancer. 2019;26(3):290–304. doi: 10.1007/s12282-018-0923-z. [DOI] [PubMed] [Google Scholar]
- 27.Corvò R, Lamanna G, Vagge S, Belgioia L, Bosetti D, Aloi D, Timon G, Bacigalupo A. Once-weekly stereotactic radiotherapy for patients with oligometastases: compliance and preliminary efficacy. Tumori. 2013;99(2):159–163. doi: 10.1700/1283.14186. [DOI] [PubMed] [Google Scholar]
- 28.Bartelink H, Horiot JC, Poortmans PM, Struikmans H, Van den Bogaert W, Fourquet A, Jager JJ, Hoogenraad WJ, Oei SB, Wárlám-Rodenhuis CC, Pierart M, Collette L. Impact of a higher radiation dose on local control and survival in breast-conserving therapy of early breast cancer: 10-year results of the randomized boost versus no boost EORTC 22881-10882 trial. J Clin Oncol. 2007;25(22):3259–3265. doi: 10.1200/JCO.2007.11.4991. [DOI] [PubMed] [Google Scholar]