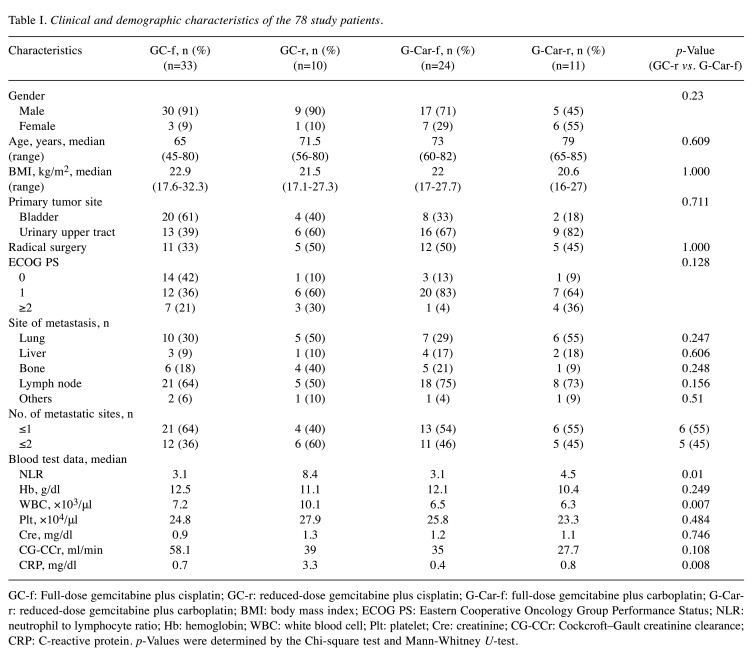
Table I. Clinical and demographic characteristics of the 78 study patients.
GC-f: Full-dose gemcitabine plus cisplatin; GC-r: reduced-dose gemcitabine plus cisplatin; G-Car-f: full-dose gemcitabine plus carboplatin; G-Carr: reduced-dose gemcitabine plus carboplatin; BMI: body mass index; ECOG PS: Eastern Cooperative Oncology Group Performance Status; NLR: neutrophil to lymphocyte ratio; Hb: hemoglobin; WBC: white blood cell; Plt: platelet; Cre: creatinine; CG-CCr: Cockcroft–Gault creatinine clearance; CRP: C-reactive protein. p-Values were determined by the Chi-square test and Mann-Whitney U-test.