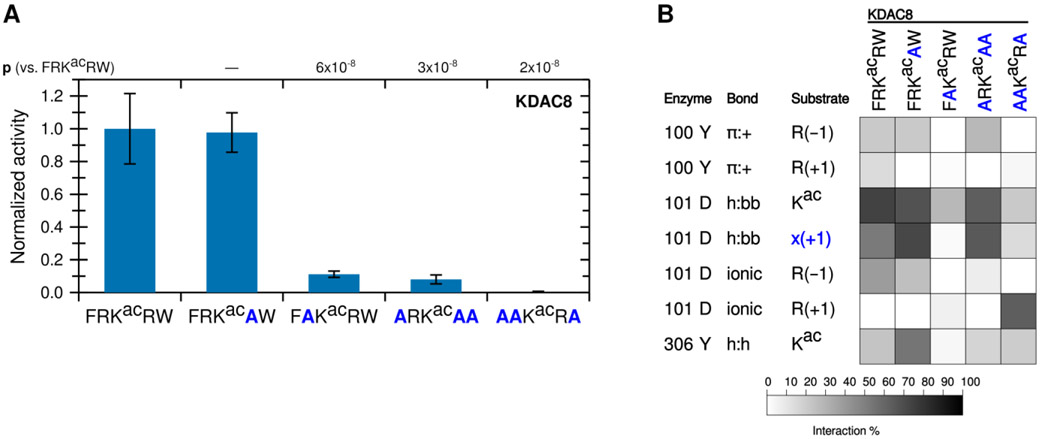
Figure 2. The −1 substrate position participates in an ionic interaction with KDAC8.
(A) Peptides derived from FRKacRW, where residues were substituted with alanine, were reacted with KDAC8. Average specific activity was normalized such that the activity of KDAC8 with FRKacRW was represented as 1. Error bars represent standard deviations (n≥4), and p-values are shown for statistically significant differences between activity with FRKacRW and activity with each other substrate. (B) Results of MD analysis identifying interactions between specific residues of KDAC8 and the derivative peptide substrates reacted in panel A. x(+1) refers to the substrate residue in the +1 position for each peptide. Shading corresponds to the percent of time a particular interaction was observed during MD simulations.