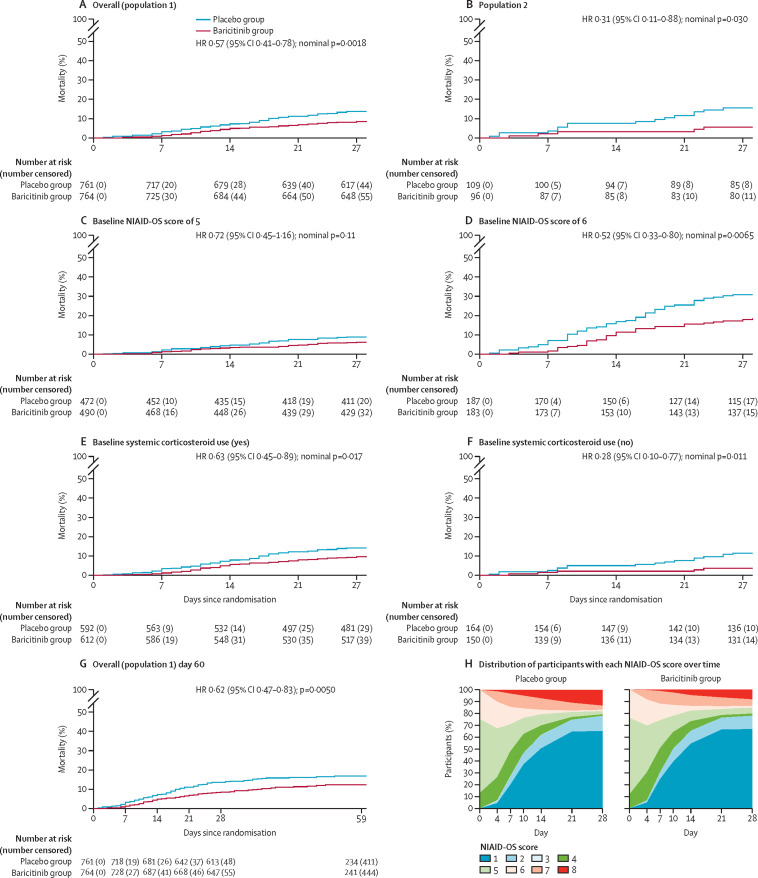
Figure 2.
Kaplan-Meier estimates of 28-day and 60-day all-cause mortality, and distribution of participants with each NIAID-OS score over time
(A–F) 28-day all-cause mortality in population 1, the overall population (A); population 2, comprising participants who, at baseline, required oxygen supplementation and were not receiving dexamethasone or other systemic corticosteroids for the primary study condition (B); populations with baseline NIAID-OS scores of 5 (C) or 6 (D); and populations with (E) and without (F) baseline systemic corticosteroid use. The number at risk at day 27 represents the number of participants with available data at day 28. (G) 60-day all-cause mortality in population 1. The number at risk and number censored before day 28 differ slightly between panels A and G because the day 60 database contained further information on eight participants who were censored at the day 28 database lock but were known to be alive at the day 60 database lock. The number at risk at day 59 represents the number of participants with available data at day 60. For time-to-event endpoints, the p value for baricitinib versus placebo was calculated using an unstratified log-rank test, and HRs and 95% CIs were calculated using a Cox proportional hazards model. The treatment effect was adjusted by all baseline randomisation factors, except when redundant (ie, for baseline corticosteroid use in population 2). (H) Distribution of participants in each NIAID-OS category over time, among patients in the intention-to-treat population with available baseline NIAID-OS scores and at least one post-baseline NIAID-OS score, using last observation carried forward. An NIAID-OS score of 5 represents patients who are hospitalised and require supplemental oxygen, and a score of 6 represents patients who are hospitalised and receiving oxygen support via high-flow oxygen devices or non-invasive ventilation. HR=hazard ratio. NIAID-OS=National Institute of Allergy and Infectious Disease Ordinal Scale.