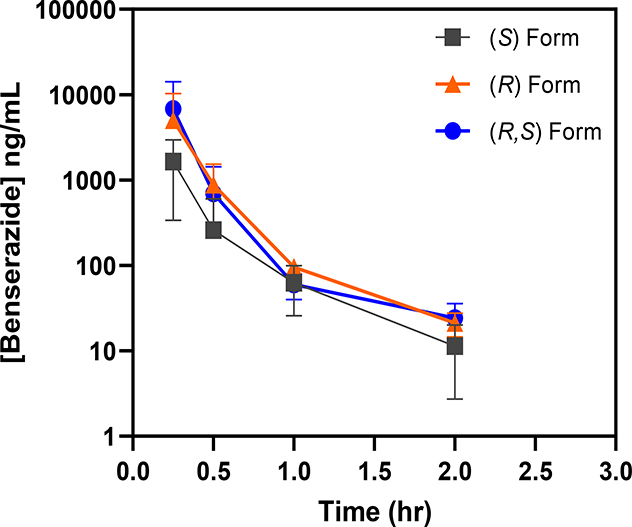
Figure 4. Similar plasma exposure profile exhibited by racemic benserazide and its enantiomers.
After completion of the efficacy portion of the study, the assigned β-YAC mice now 10–12 weeks old were treated 2 days later with a single 20 mg/kg dose of the racemic (R,S)-benserazide HCL (EEY), (R)-benserazide HCL (EF2) and (S)-benserazide HCL (EF3) drug formulations. Blood samples were collected at designated timepoints and drug concentrations were analyzed as described (See Methods). Shown are the drug concentration-time curves for animals administered the (R)-, (S)- and (R,S)-benserazide.