Figure 1.
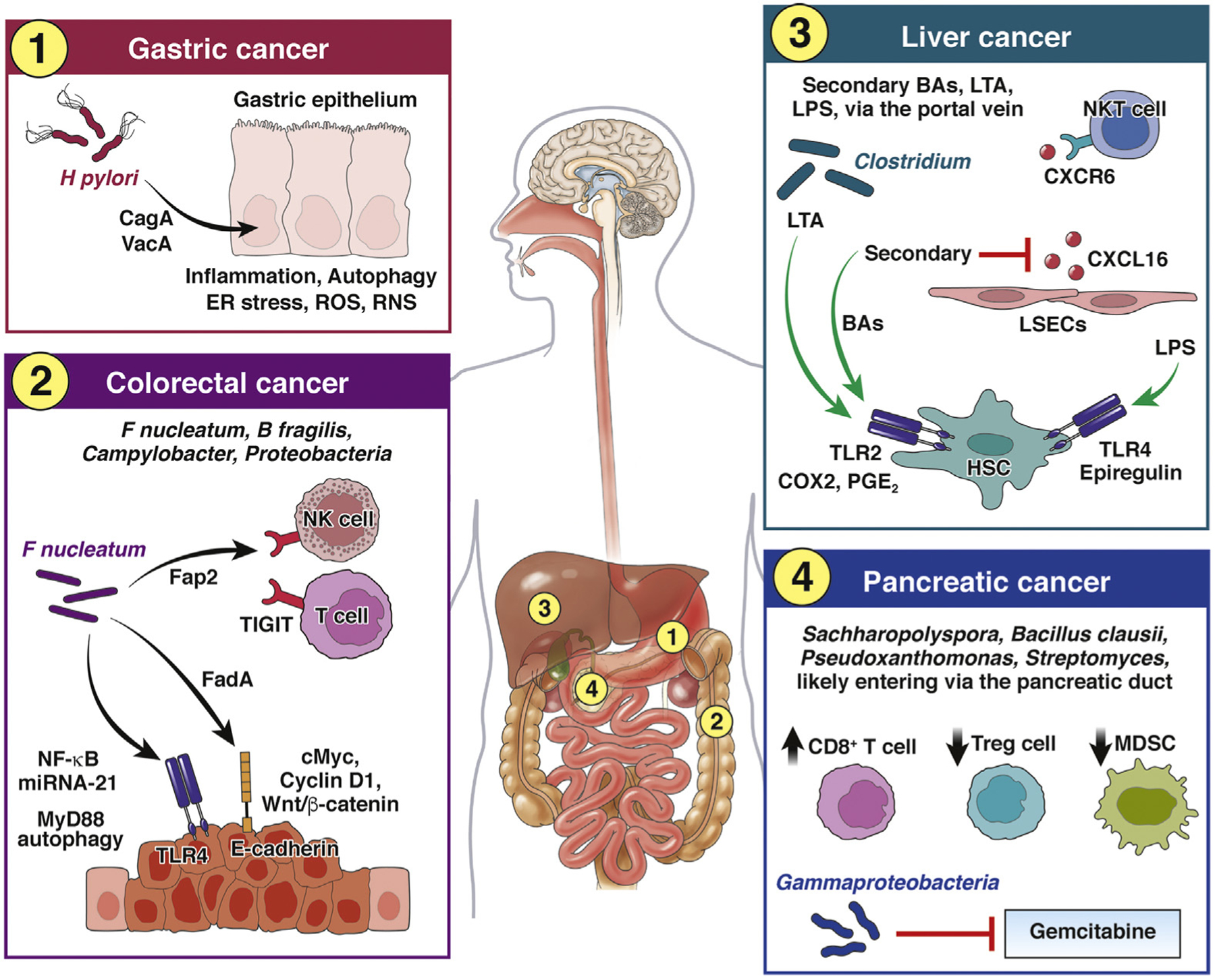
Local effects of the gut microbiota on tumor development. Gastric cancer: H pylori release virulence factors (eg, CagA, VacA) causing endoplasmic reticulum (ER) stress, autophagy, and oxidative stress in gastric epithelial cells, which collectively contribute to cancer development. RNS, reactive nitrogen species; ROS, reactive oxygen species. Colorectal cancer: F nucleatum contributes to tumor development and progression via several mechanisms. The virulence factor FadA can signal through E-cadherin and lead to an increase in annexin A1 expression, activation Wnt/β-catenin signaling, and up-regulation oncogenes c-Myc and cyclin D1. Signaling through TLR4 activates of NF-κB and up-regulation of microRNA-21 (miRNA-21), which also has oncogene functions. Signaling through TLR4 also induces MyD88-driven autophagy in cancer cells, thus aiding in chemoresistance. The outer membrane protein Fap2 binds to inhibitory TIGIT receptor on tumor-infiltrating NK cells and T cells, thus aiding in cancer immune evasion. Other gut bacteria, such as B fragilis, Campylobacter, and Proteobacteria, have also been associated with the development of CRC. Liver cancer: Gut bacterial metabolites (eg, secondary BAs) and microbe-associated molecular patterns, e.g., LTA and LPS) enter the liver via the hepatic portal vein and exert diverse effects on various cells in the liver that collectively contribute to cancer development and immune evasion. LPS-driven TLR4 signaling up-regulates the hepatomitogen epiregulin in hepatic stellate cells (HSCs), which can contribute to cancer development. Enrichment of Clostridium can result in accumulation of LTA and of the secondary BA deoxycholine, which collectively signal via TLR2 and up-regulate COX2, causing increase in prostaglandin E2 (PGE2), which in turn inhibits tumor cell killing by infiltrating CD8+ T cells, thus contributing to cancer immune evasion. Secondary BAs can also inhibit secretion of CXCL16 by liver sinusoidal endothelial cells (LSECs) required for recruitment of NKT cells, thus further contributing to cancer immune evasion. Pancreatic cancer: Gut bacteria found in pancreatic tumors, such as Bacillus clausii, Sachharopolyspora, Streptomyces, and Pseudoxanthomonas, are associated with improved antitumor immunity in pancreatic cancer, and Gammaproteobacteria contribute to resistance to gemcitabine chemotherapy by metabolizing the active form of the drug. The route of translocation from the gut to the pancreas is likely via the pancreatic duct, which opens into the duodenum. MDSC, myeloid-derived suppressor cells.
