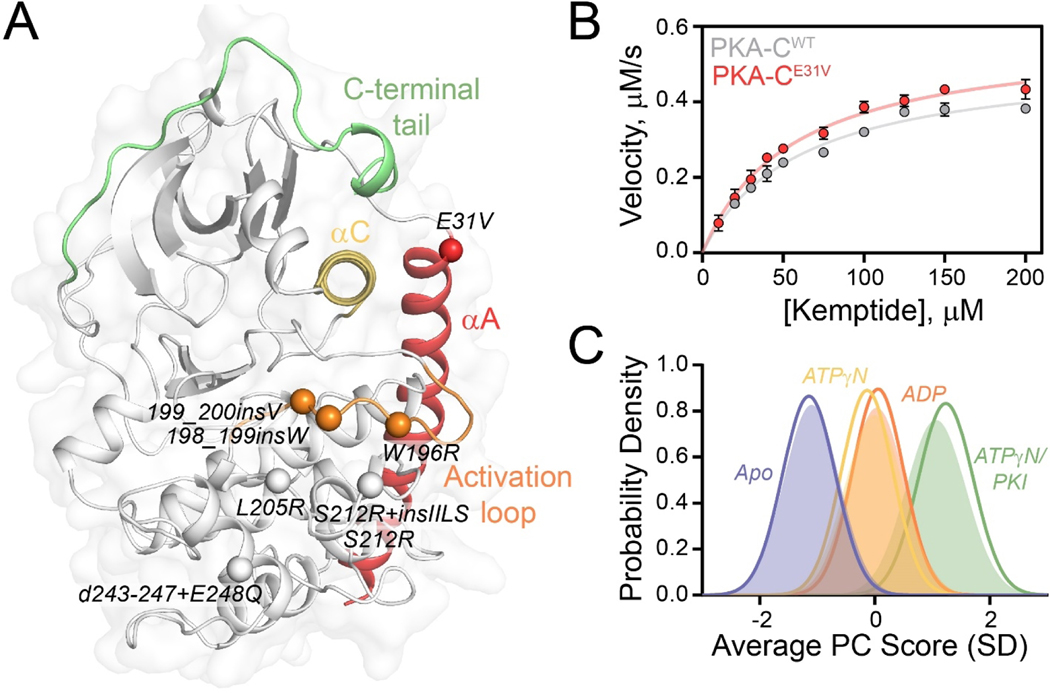
Figure 1. Structural and kinetic characterization of PKA-CE31V.
(A) X-ray structure of PKA-C bound to endogenous inhibitor, PKI (PDB: 1ATP) highlighting important structural elements and locations of Cushing’s syndrome mutations in relation to E31V. (B) Steady state phosphorylation kinetics of PKA-CE31V with Kemptide. (C) CONCISE analysis on the apo, ATPγN-, ADP- and ATPγN/PKI-bound forms of PKA-CWT (opaque gaussian) and PKA-CE31V (outlined gaussian). Note that the probability distribution of PKA-CWT when bound to ATPγN (yellow) and ADP (orange) are overlapping.