An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.
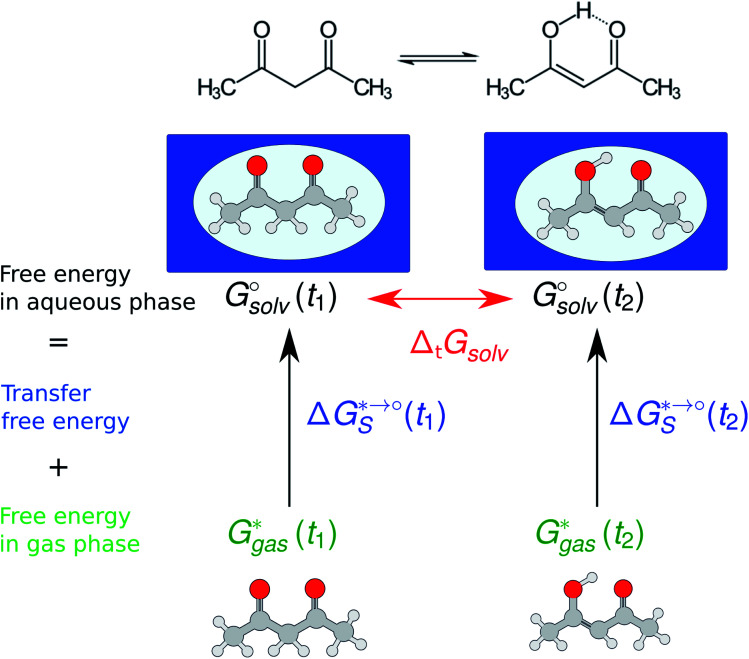
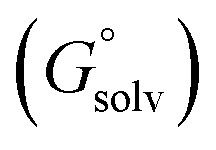 as the sum of the free energy in gas phase (
as the sum of the free energy in gas phase (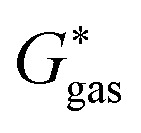 ; calculated using the ideal gas RRHO approximation) and the standard state transfer free energy (
; calculated using the ideal gas RRHO approximation) and the standard state transfer free energy (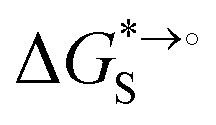 ; obtained using a continuum solvation model). The tautomeric free energy difference in solution
; obtained using a continuum solvation model). The tautomeric free energy difference in solution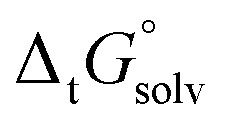 is then calculated as the difference between
is then calculated as the difference between 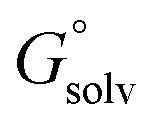 for two tautomers.
for two tautomers.