Figure 5. Engineered vector may delay, but does not prevent, intraocular inflammation following intravitreal AAV2 administration in NHPs.
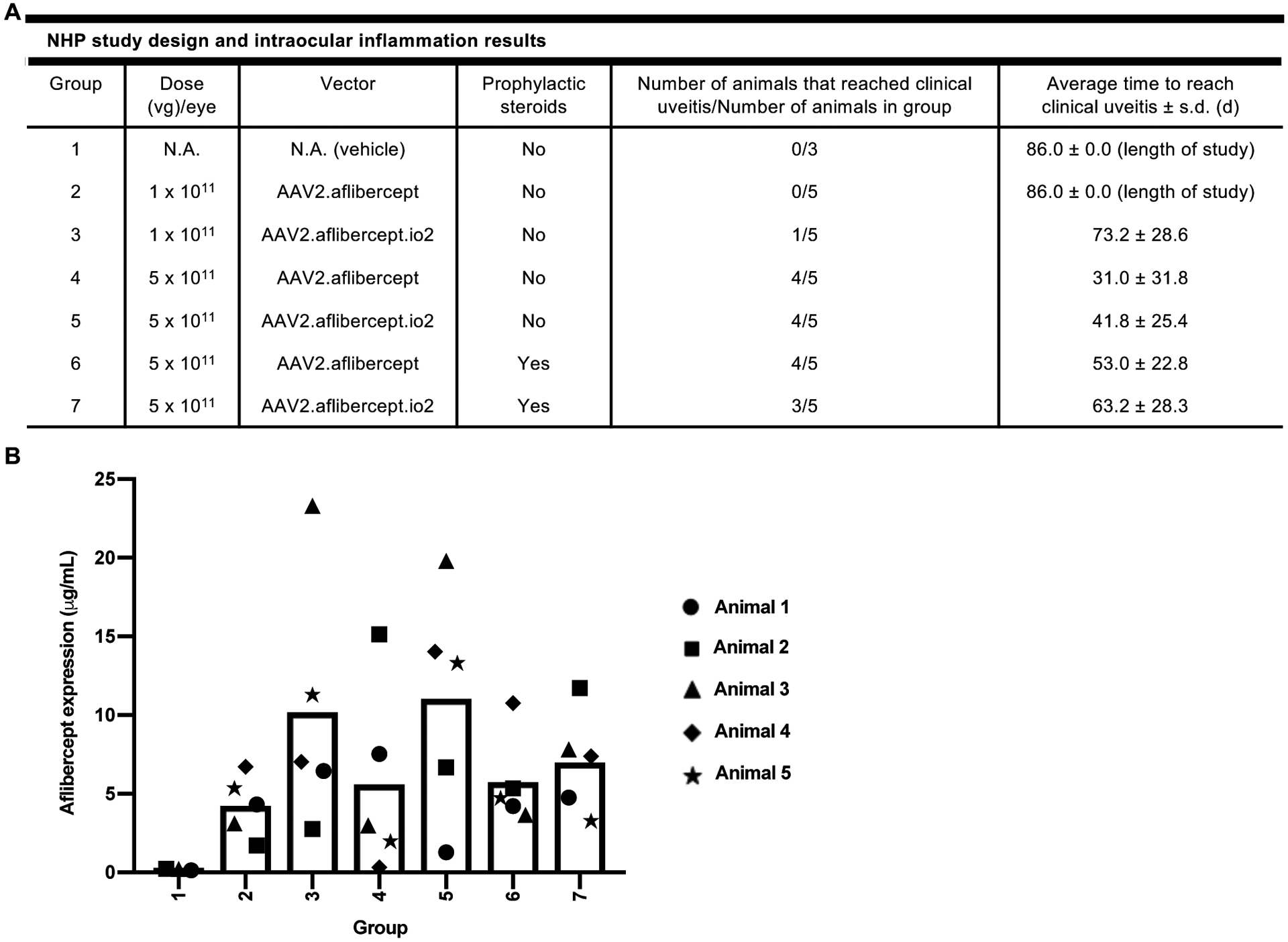
(A) Summary of NHP study design and key intraocular inflammation results. The indicated number of NHPs received bilateral intravitreal injections of AAV2.aflibercept or AAV2.aflibercept.io2 at 1 × 1011 vg or 5 × 1011 vg on Day 1, and intraocular inflammation was scored based on the SUN criteria system during the 12-week study. Group 6 and 7 animals received intramuscular injection of prophylactic systemic steroids on Days −1 and 6. Clinical uveitis was defined as AC or VC score of 3 or higher. A two-tailed Fisher’s Exact Test with stepdown Sidak adjustment was used to analyze number of animals that reached clinical uveitis while a two-tailed generalized linear model was used to analyze average time to reach clinical uveitis. No statistically significant differences were detected between groups. (B) Aflibercept concentrations (μg/mL) in NHP vitreous humors. Aflibercept concentrations were measured in NHP vitreous humor samples collected 12 weeks post-injection. For AAV-treated animals, animals 1 and 2 were male and animals 3 to 5 were female. For vehicle-treated animals, animal 1 was male and animals 2 and 3 were female. A univariate linear regression model with aflibercept expression as the outcome was used to compare vitreous aflibercept concentrations in vector-injected groups. No statistically significant differences were detected between groups.
