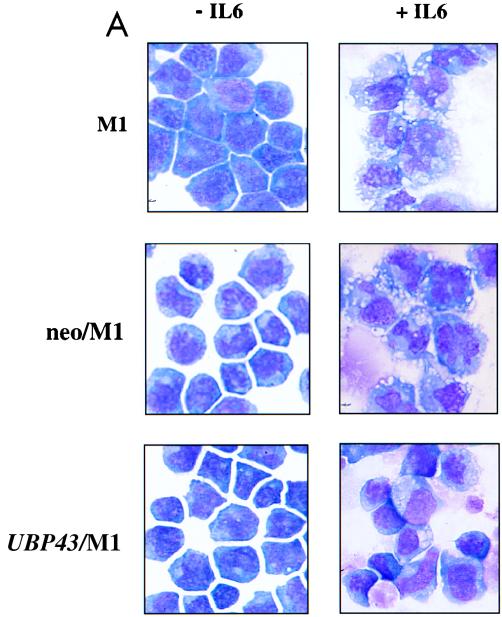
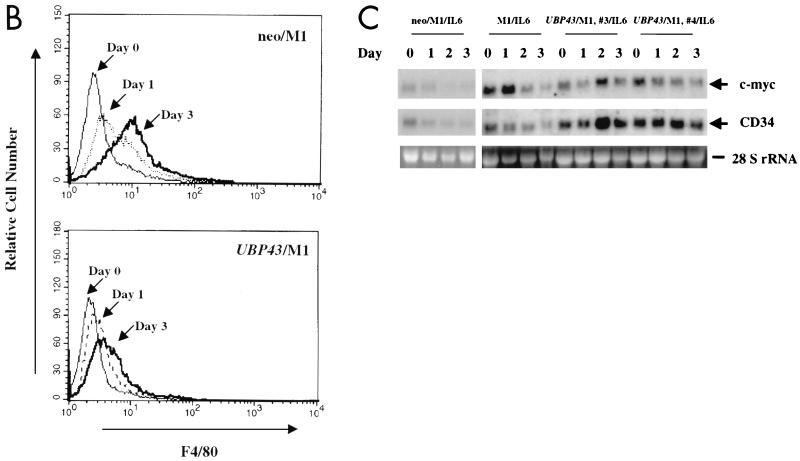
FIG. 9.
Constitutive expression of UBP43 in M1 cells blocks terminal macrophage differentiation. (A) Morphological analysis. After 3 days of culture in the absence or presence of IL-6, cells were evaluated for morphological evidence of terminal differentiation. As detected by Wright-Giemsa stain, the majority of parental M1 cells or control M1 cells infected with retroviral vector alone (neo/M1) showed the morphological features of mature macrophages: heavily vacuolated cytoplasm, a reduced nuclei to cytoplasmic ratio, distinct heterochromatin, and the lack of prominent nucleoli. In contrast, cells overexpressing UBP43 (UBP43/M1) showed evidence of arrested maturation, with most cells having the morphology of promonoblasts or undifferentiated blasts. UBP43-overexpressing cells that were not treated with IL-6 remained entirely undifferentiated, similar to parental and control cells. (B) Flow cytometry analysis of F4/80 expression on IL-6 treated control M1 cells (neo/M1) and UBP43-overexpressing M1 cells (UBP43/M1) by using a phycoerythrin-conjugated monoclonal antibody specific for F4/80. Results from representative clones are shown. (C) Northern blot analysis of CD34 and c-myc expression. Total RNA from M1, neo/M1, and UBP43/M1 clone 3 as well as UBP43/M1 clone 4 cells cultured for the times indicated in the presence of 100 ng of IL-6 per ml were prepared for analysis by Northern blotting of the expression of c-myc and CD34. Each lane was loaded with 10 μg of total RNA. The blot was hybridized with a 32P-labeled murine c-myc or murine CD34 cDNA probe. Ethidium bromide-stained 28S rRNA is shown to demonstrate equivalent RNA loading.