Figure 12. Intravenously administered recombinant human (h) NGAL increases in the urine of mice with maleic acid administration and ischemic AKI to a greater extant than during pre-renal azotemia (furosemide administration).
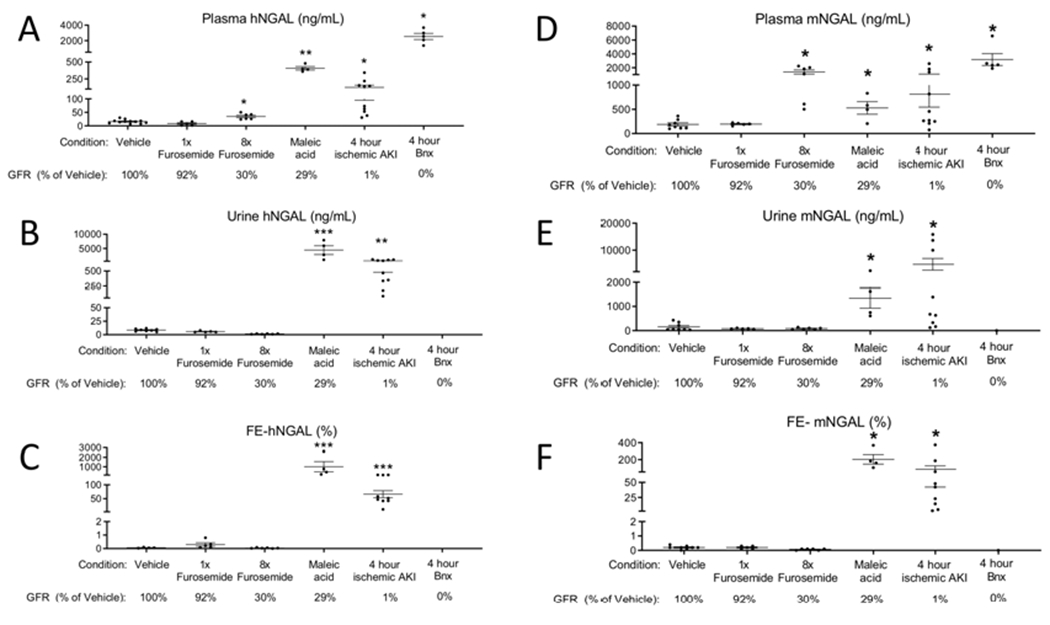
rhNGAL was administered intravenously(IV) 6h after vehicle, furosemide, and maleic acid; and 4 hours ischemic AKI and bilateral nephrectomy (BNx). Endpoints were determined 1 after IV hNGAL administration. (A) hNGAL was significantly increased in the plasma after severe prerenal azotemia (8x furosemide), maleic acid administration, ischemic AKI, and bilateral nephrectomy versus vehicle. (B) Urine hNGAL was significantly increased after maleic acid treatment and ischemic AKI versus vehicle. (C) Fractional excretion (FE) of hNGAL was less than 1% in vehicle, mild prerenal azotemia (1x furosemide), and severe prerenal azotemia (8x furosemide), and was greater than 10% after maleic acid treatment and ischemic AKI. (D-F) Endogenous murine (m) NGAL was determined in the plasma and urine, and FE-mNGAL was calculated. Analyzed by t test versus vehicle, *P<0.05, **P<0.01, ***P<0.001. n=5-12 (groups include mice that directly had GFR measured, as well as additional mice; GFR for each model relative to vehicle is indicated below for reference).
