Figure 5.
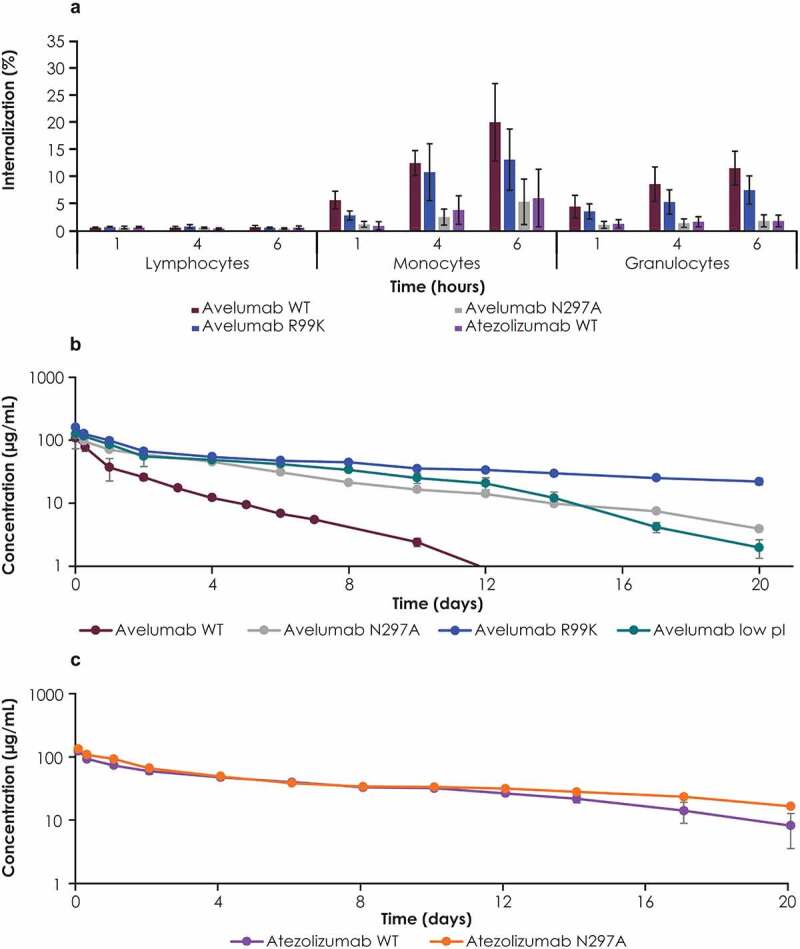
Studies with PD-L1 antibodies and their variants in cynomolgus monkeys. (a) Internalization of avelumab and its FcγR binding–deficient (N297A) and PD-L1 binding–deficient (R99K) variants was assessed in whole blood samples from cynomolgus monkeys. Serum concentration profiles of (b) WT avelumab, n = 2; avelumab FcγR binding–deficient (N297A), n = 1; avelumab PD-L1 binding–deficient (R99K), n = 3; avelumab low pI: n = 3; and (c) WT atezolizumab with, n = 2 and atezolizumab N297A (FcγR binding-deficient), n = 1. Cynomolgus monkeys were dosed with 5 mg/kg of antibody variants (WT avelumab was dosed at 4 mg/kg and normalized to 5 mg/kg). Serum concentrations were measured by immunoassay; profiles affected by antidrug antibodies were excluded. Error bars represent standard deviations. FcγR, Fcγ receptor; pI, isoelectric point; WT, wild-type
