Rheumatology key message
An XWAS revealed that variations in the X chromosome influence serum urate levels.
Dear Editor, we have recently identified 36 genomic loci influencing serum urate (SU) levels via a genome-wide meta-analysis using 121,745 Japanese individuals [1]; however, non-autosomal data have hitherto been excluded in genome-wide association study (GWAS) quality-control procedures. The same limitation was applied to a trans-ancestry GWAS of SU in 457,690 individuals, the largest currently published genetic study on SU [2]. Other previous GWASs [3] have focussed exclusively on the associations between genetic variants in the autosomes and SU. The largely unknown role of the X chromosome in SU levels, in spite of it constituting ∼5% of the human nuclear genome, prompted us to conduct an X chromosome-wide meta-analysis. We herein report our results, which reveal a novel locus that influences SU. Our findings may provide insights into gender differences in the homeostasis of urate, the circulating form of uric acid.
To investigate the contribution of genetic variations in the X chromosome to SU levels, we conducted an X chromosome-wide meta-analysis for SU using enlarged data sets encompassing 142,121 Japanese subjects based on three Japanese cohorts: the Japan Multi-institutional Collaborative Cohort Study, the Kita-Nagoya Genomic Epidemiology Study and the BioBank Japan Project. Details of these cohorts and analysis methods are described in the Supplementary Methods, available at Rheumatology online. Via our meta-analysis for SU, we successfully identified 12 single nucleotide polymorphisms (SNPs) at the Xq28 locus that satisfied a genome-wide significance threshold of α = 5.0 × 10−8 (Fig. 1A, supplementary Table S1, available at Rheumatology online). Strong linkage disequilibrium among these 12 SNPs was observed in the Japanese population. We first identified a novel non-autosomal locus that is associated with SU. A regional association plot for this locus is shown in Fig. 1B, where the lead SNP (rs3020789) with the lowest P-value was in an intergenic region between LOC105373383 and dual specificity phosphatase 9 (DUSP9). The results of sex-stratified analyses with the lead SNP both in each cohort and the meta-analysis are summarized in supplementary Table S2, available at Rheumatology online. In the meta-analysis, the rs3020789 showed a genome-wide significant association and a nominally significant association for the male group (β = –0.020; P = 2.12 × 10−8) and female group (β = –0.012; P = 0.024).
Fig. 1.
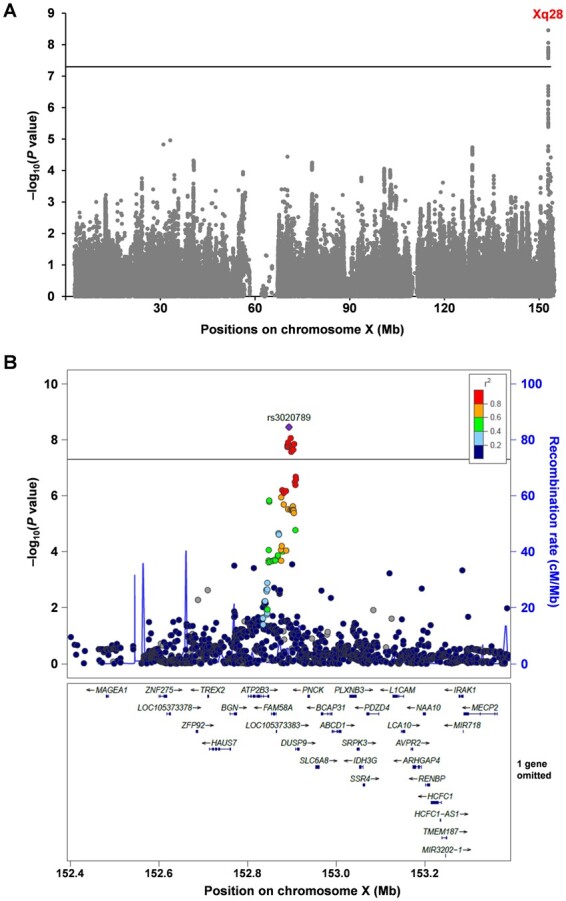
An X chromosome-wide meta-analysis revealed genetic loci influencing serum urate levels in humans
(A) Manhattan plot of the meta-analysis that combines males and females. Supplementary results for males and females alone are shown in supplementary Fig S1A and B, available at Rheumatology online, respectively. (B) Regional association plot for the Xq28 locus identified in the meta-analysis. The vertical axis indicates the –log10(P-value) for the assessment of the association of each SNP with serum urate (SU) levels. The colours indicate the linkage disequilibrium (r2) between each lead SNP and neighbouring SNPs based on female subjects of the JPT population (Japanese in Tokyo) in the 1000 Genomes Project Phase 3 (https://www.internationalgenome.org/home). Horizontal lines represent the genome-wide significance threshold (α = 5 × 10−8). The regional plot was drawn using LocusZoom (http://locuszoom.org/).
To enhance understanding of the functional annotation of the newly identified locus (Xq28) in the context of SU, we then performed further analyses using publicly available data in the Genotype-Tissue Expression database (GTEx). We found that among the 12 SNPs associated with SU at the locus, 10 SNPs harbour variants that affect the expression of at least one gene in more than one tissue in humans (supplementary Table S3, available at Rheumatology online). Based on the results of expression quantitative trait loci (eQTL) analysis, these SNPs were associated with the expression levels of their near genes [DUSP9, family with sequence similarity 58 member A (FAM58A, also known as cyclin Q—CCNQ), biglycan (BGN) or/and SRSF protein kinase 3 (SRPK3)]. Interestingly, with DUSP9, significant associations were confirmed in many tissues, including subcutaneous adipose tissue, ovary and testis. These results support the contention that DUSP9 is the most plausible gene for the effects of the identified non-autosomal variations on SU levels.
Given the well-characterized role of DUSP9 as a cytosolic mitogen-activated protein kinase phosphatase (MKP) that regulates intracellular signalling pathways [4], DUSP9 is not likely to be directly involved in SU regulation via the metabolism or transport of urate. Of note, the rs5945326 near DUSP9 has reportedly been identified as a type 2 diabetes susceptibility locus [5]. Its risk allele (rs5945326-A) was associated with SU-lowering in this study. DUSP9 is expressed in insulin-sensitive tissues and the kidney (a dominant tissue for urate elimination) [6]. Thus, besides its protective effect against the development of insulin resistance [4], DUSP9 may influence renal functions via a different mechanism. Although our eQTL analysis found little effect of the identified SNPs on DUSP9 levels in the kidney (possibly due to a lack of information on the kidney in GTEx), finding out how, with or without inter-organ communication, DUSP9 might be involved in renal urate handling is likely to be significant and merits further study.
In this study we provide genetic evidence showing the SU-influencing effects of non-autosomal variants. Interestingly, for the lead SNP, its effect allele (rs3020789-T) frequencies in various populations range from 0.480 to 0.805 (supplementary Table S4, available at Rheumatology online). Whether a similar association can be found in other populations, as in this study on a Japanese population, should therefore be addressed. Considering that this association was been uncovered in a previous study that consisted of a GWAS for 58 quantitative traits including SU using autosomal and X chromosome variants [7], and the fact that knowledge of sex chromosomes lags behind that of autosomal chromosomes in population genetics research [8], our findings may contribute to a better understanding of the genetic factors that might be associated with gender differences in SU levels, and open a path to elucidating the physiological regulatory mechanisms of SU in humans.
Ethics statement
Data and sample collection for the cohorts participating in the present study were approved by the respective research ethics committees (National Defense Medical College; Osaka University; Nagoya University; RIKEN; Jichi Medical University). All studies were performed according to the guidelines of the declaration of Helsinki. All participants had provided their written informed consent.
Supplementary Material
Acknowledgements
We would like to thank all the participants for their generous involvement in this study. We also thank M. Miyazawa, K. Morichika and M. Seki (National Defense Medical College) for technical assistance. M.N., Y.O. and H. Matsuo conceived and designed the study; Y.T., A.N., Y. Kawamura, and N.S. assisted with research design; M.N., A.H., H. Mikami, K.M., T.T., Y.M., Y. Kamatani, S.I., M.Y. and K.W. collected and/or analysed clinical data; M.N., M.K., Y. Kamatani and Y.O. performed the statistical analysis; M.N., Y.T., A.N., Y. Kawamura and H. Matsuo analysed the data; H. Matsuo organized this collaborative study; M.K., A.N., Y. Kawamura, A.H., N.S., M.Y., K.W. and Y.O. provided intellectual input and assisted with the preparation of the manuscript; M.N., Y.T. and H. Matsuo wrote the manuscript.
Collaborators: Members of the Japan Uric Acid Genomics Consortium (Japan Urate) are: Kimiyoshi Ichida, Tappei Takada, Seiko Shimizu, Kenji Takeuchi, Kiyonori Kuriki, Sadao Suzuki, Yoshikuni Kita, Ritei Uehara, Kokichi Arisawa, Hiroaki Ikezaki, Keitaro Tanaka, Ken Yamamoto, Tomoko S. Kato, Tatsuaki Matsubara, Yuka Aoki, Takahiro Nakamura, Hiroshi Nakashima and Masashi Tsunoda.
Funding: This study was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI [grant numbers 17015018, 221S0001, 16H06277 (CoBiA), 17H04128, 17K19864, 19K19434, 19K22786, 20H00566, 20K23152 and 21H03350] as well as a research grant from the Gout and uric acid foundation of Japan. This study was supported in part by funding from the BioBank Japan Project for the Japan Agency for Medical Research and Development, and the Ministry of Education, Culture, Sports, Science and Technology (MEXT). This study was also supported in part by Grants-in-Aid from MEXT (grant numbers 24390169, 25293144, 16H05250 and 20K10514) as well as by a grant from the Funding Program for Next-Generation World-Leading Researchers (NEXT Program, no. LS056).
Disclosure statement: The authors declare that they have no conflict of interest.
Data availability statement
Data are available upon reasonable request.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1.Nakatochi M, Kanai M, Nakayama A. et al. Genome-wide meta-analysis identifies multiple novel loci associated with serum uric acid levels in Japanese individuals. Commun Biol 2019;2:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tin A, Marten J, Halperin Kuhns VL. et al. Target genes, variants, tissues and transcriptional pathways influencing human serum urate levels. Nat Genet 2019;51:1459–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Major TJ, Dalbeth N, Stahl EA, Merriman TR.. An update on the genetics of hyperuricaemia and gout. Nat Rev Rheumatol 2018;14:341–53. [DOI] [PubMed] [Google Scholar]
- 4.Emanuelli B, Eberlé D, Suzuki R, Kahn CR.. Overexpression of the dual-specificity phosphatase MKP-4/DUSP-9 protects against stress-induced insulin resistance. Proc Natl Acad Sci USA 2008;105:3545–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Voight BF, Scott LJ, Steinthorsdottir V. et al. ; The MAGIC investigators. Twelve type 2 diabetes susceptibility loci identified through large-scale association analysis. Nat Genet 2010;42:579–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu H, Dembski M, Yang Q. et al. Dual specificity mitogen-activated protein (MAP) kinase phosphatase-4 plays a potential role in insulin resistance. J Biol Chem 2003;278:30187–92. [DOI] [PubMed] [Google Scholar]
- 7.Kanai M, Akiyama M, Takahashi A. et al. Genetic analysis of quantitative traits in the Japanese population links cell types to complex human diseases. Nat Genet 2018;50:390–400. [DOI] [PubMed] [Google Scholar]
- 8.Editorial. Accounting for sex in the genome. Nat Med 2017;23:1243. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request.


