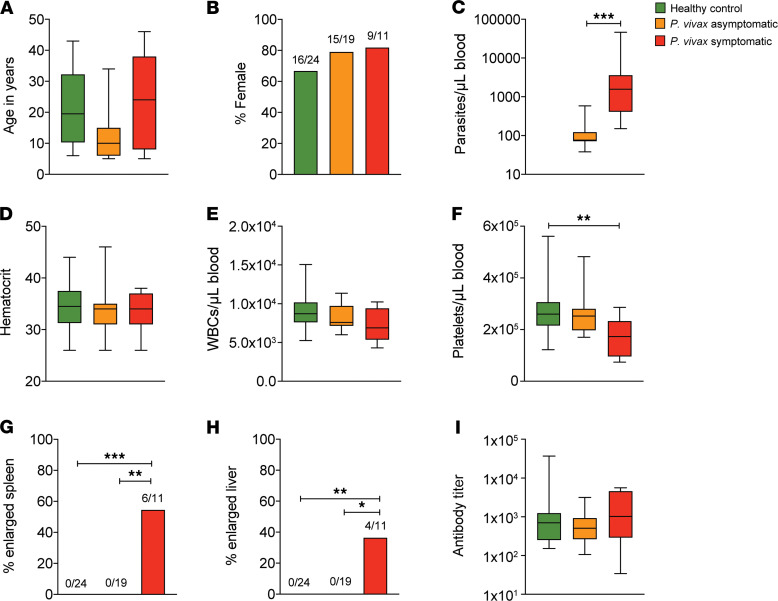
Figure 1. Study cohort characteristics.
P. vivax symptomatic (n = 11) and asymptomatic (n = 19) infected individuals as well as healthy immune controls (n = 24) were selected for analysis of immune responses to infection. Age (A) and sex (B) were not different while parasitemia was significantly higher in the symptomatic group (C). Other clinical parameters determined in the study include hematocrit (D), WBC count (E), platelet count (F), as well as the proportion of participants presenting with enlarged spleen (G) or liver (H). Antibody titers specific for P. vivax recombinant DBP were determined by ELISA (I). Boxes represent the 25th to 75th percentile, whiskers show the range (minimum to maximum), and lines represent the median. *P < 0.05, **P < 0.01, ***P < 0.001. Significance was determined by Kruskal-Wallis with Dunn’s multiple-comparison test (A, D, E, F, and I), Mann-Whitney test (C), or Fisher’s exact test (B, G, and H).