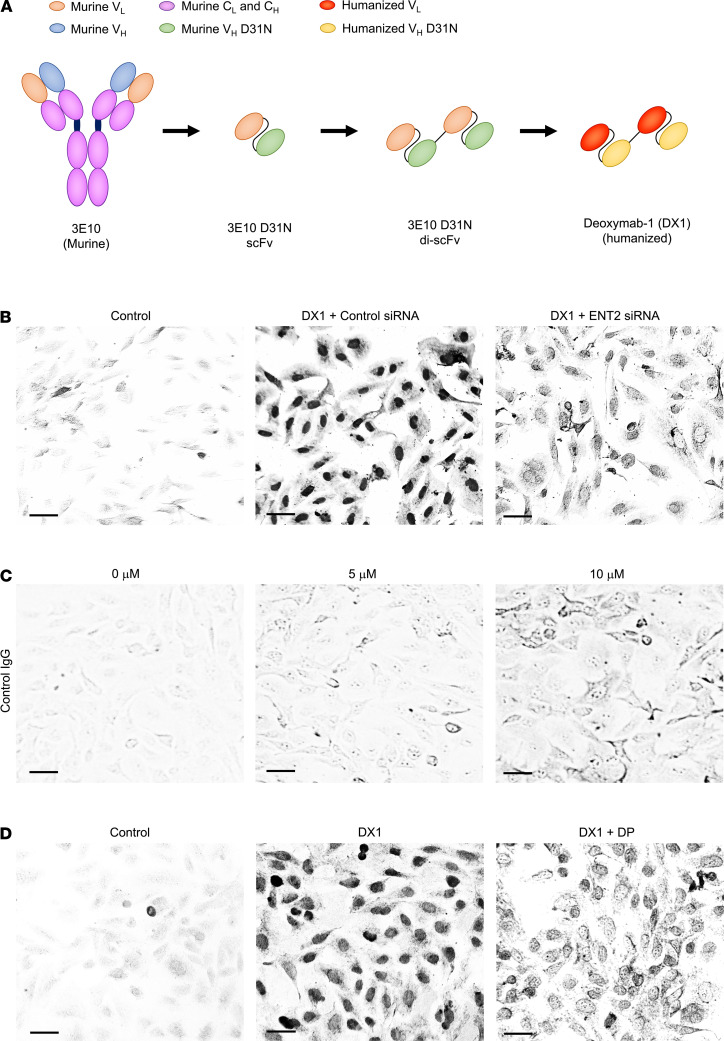
Figure 1. DX1 penetration into human brain endothelial cells is dependent on ENT2.
(A) Illustrated evolution of 3E10 into DX1. 3E10 was isolated from the MRL/lpr lupus mouse model. A 3E10 single chain variable fragment (scFv) with D31N mutation in the heavy chain variable domain complementarity determining region 1 (VH CDR1) was previously shown to have higher affinity for DNA and efficiency of cellular penetration compared with the original 3E10. 3E10 D31N di-scFv has greater impact on the DNA damage response and synthetic lethality to PTEN-deficient cancer cells due to its increased avidity for DNA compared with the scFv. The 3E10 D31N di-scFv was humanized, deimmunized, and CDR-optimized to yield DX1, which is now in development for testing in clinical trials (8, 17). (B) DX1 penetrates into brain endothelial cells in an ENT2-dependent manner. hCMEC/D3 cells transfected with control or ENT2-targeting siRNA were treated with control buffer or DX1 alone, and then stained to detect DX1 penetration. Representative images are shown. Scale bar: 30 μm. (C) Control IgG shows minimal uptake into brain endothelial cells. hCMEC/D3 cells were treated with 0–10 μM of a control IgG (specifically an anti-PD1 antibody) and stained to detect uptake of IgG. Representative images are shown. Scale bar: 30 μm. (D) The ENT2 inhibitor DP interferes with DX1 penetration into brain endothelial cells. hCMEC/D3 cells were treated with control buffer, DX1, or DX1 + 50 μM DP and stained for DX1. Representative images are shown. Scale bar: 30 μm. These data demonstrate ENT2-dependent penetration by DX1 into hCMEC/D3 cells.