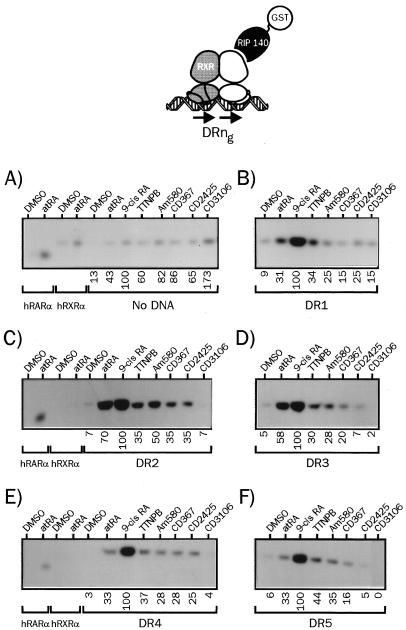
FIG. 4.
The nature of the RARE determines the relative affinity of liganded heterodimers for nuclear coactivators. (A) 35S-labeled hRXRα and hRARα produced by coupled in vitro transcription-translation were incubated in the presence of the indicated retinoids at 10 μM (final concentration), and complexes were bound to a GST-RIP140 fusion protein. After a 2-h incubation, complexes were isolated by adsorption on a glutathione-linked agarose matrix and analyzed by SDS-PAGE (8% gel). (B to F) hRXRα-hRARα heterodimers associated with the indicated DRng RARE were analyzed for the ability to interact with RIP140 in the presence of natural or synthetic retinoids as described above. The nature of the response element is indicated below each panel. Numbers below gel lanes indicate the amount of radiolabeled receptor bound to RIP140 in the presence of the indicated ligand relative to that measured in the presence of 9-cis RA, defined as 100%. Values represent mean data from four independent experiments carried out with two different bacterial extracts. Standard errors never exceeded 6.2%. Representative autoradiograms are shown.